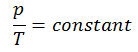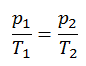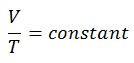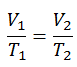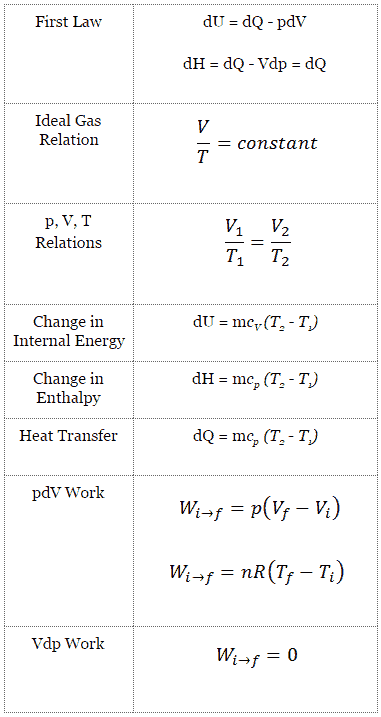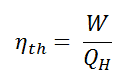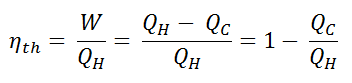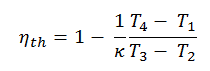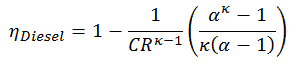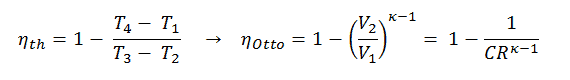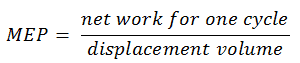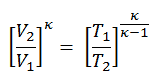Diesel Cycle – Diesel Engine
In the 1890s, a German inventor, Rudolf Diesel has patented his invention of an efficient, slow burning, compression ignition, internal combustion engine. The original cycle proposed by Rudolf Diesel was a constant temperature cycle. In later years Diesel realized his original cycle would not work and he adopted the constant pressure cycle, which is known as the Diesel cycle.
Diesel cycle is one of most common thermodynamic cycles that can be found in automobile engines and describes the functioning of a typical compression ignition piston engine. The Diesel engine is similar in operation to the gasoline engine. The most important difference is that:
- There is no fuel in the cylinder at the beginning of the compression stroke, therefore an autoignition does not occur in Diesel engines.
- Diesel engine uses compression ignition instead of spark ignition.
- Because of the high temperature developed during the adiabatic compression, the fuel ignites spontaneously as it is injected. Therefore no spark plugs are needed.
- Before the beginning of the power stroke, the injectors start to inject fuel directly into the combustion chamber and therefore first part of power stroke occurs approximately at the constant pressure.
- Higher compression ratios can be achieved in Diesel engines, than in Otto engines
Since Carnot’s principle states that no engine can be more efficient than a reversible engine (a Carnot heat engine) operating between the same high temperature and low temperature reservoirs, the Diesel engine must have lower efficiency than the Carnot efficiency. A typical diesel automotive engine operates at around 30% to 35% of thermal efficiency. About 65-70% is rejected as waste heat without being converted into useful work, i.e. work delivered to wheels. In general, engines using the Diesel cycle are usually more efficient, than engines using the Otto cycle. The diesel engine has the highest thermal efficiency of any practical combustion engine. Low-speed diesel engines (as used in ships) can have a thermal efficiency that exceeds 50%. The largest diesel engine in the world peaks at 51.7%.
Diesel Cycle – Processes
In an ideal Diesel cycle, the system executing the cycle undergoes a series of four processes: two isentropic (reversible adiabatic) processes alternated with one isochoric process and one isobaric process.
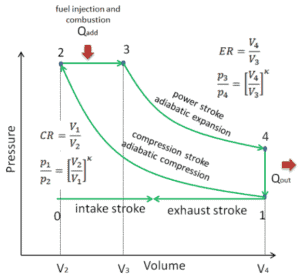
pV diagram of an ideal Diesel cycle Isentropic compression (compression stroke) – The air is compressed adiabatically from state 1 to state 2, as the piston moves from bottom dead center to top dead center. The surroundings do work on the gas, increasing its internal energy (temperature) and compressing it. On the other hand the entropy remains unchanged. The changes in volumes and its ratio (V1 / V2) is known as the compression ratio.
- Isobaric expansion (ignition phase) – In this phase (between state 2 and state 3) there is a constant-pressure (idealized model) heat transfer to the air from an external source (combustion of injected fuel) while the piston is moving toward the V3. During the constant pressure process, energy enters the system as heat Qadd, and a part of work is done by moving piston.
- Isentropic expansion (power stroke) – The gas expands adiabatically from state 3 to state 4, as the piston moves from V3 to bottom dead center. The gas does work on the surroundings (piston) and loses an amount of internal energy equal to the work that leaves the system. Again the entropy remains unchanged. The volume ratio (V4 / V3) is known as the isentropic expansion ratio.
- Isochoric decompression (exhaust stroke) – In this phase the cycle completes by a constant-volume process in which heat is rejected from the air while the piston is at bottom dead center. The working gas pressure drops instantaneously from point 4 to point 1. The exhaust valve opens at point 4. The exhaust stroke is directly after this decompression. As the piston moves from bottom dead center (point 1) to top dead center (point 0) with the exhaust valve opened, the gaseous mixture is vented to the atmosphere and the process starts anew.
During the Diesel cycle, work is done on the gas by the piston between states 1 and 2 (isentropic compression). Work is done by the gas on the piston between stages 2 and 3 (isobaric heat addition) and between stages 2 and 3 (isentropic expansion). The difference between the work done by the gas and the work done on the gas is the net work produced by the cycle and it corresponds to the area enclosed by the cycle curve. The work produced by the cycle times the rate of the cycle (cycles per second) is equal to the power produced by the Diesel engine.
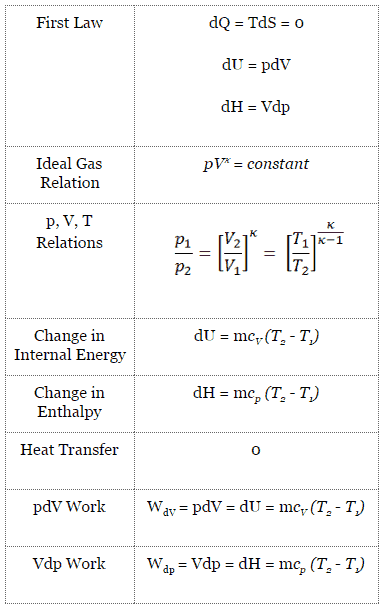
Isentropic Process
An isentropic process is a thermodynamic process, in which the entropy of the fluid or gas remains constant. It means the isentropic process is a special case of an adiabatic process in which there is no transfer of heat or matter. It is a reversible adiabatic process. The assumption of no heat transfer is very important, since we can use the adiabatic approximation only in very rapid processes.
Isentropic Process and the First Law
For a closed system, we can write the first law of thermodynamics in terms of enthalpy:
dH = dQ + Vdp
or
dH = TdS + Vdp
Isentropic process (dQ = 0):
dH = Vdp → W = H2 – H1 → H2 – H1 = Cp (T2 – T1) (for ideal gas)
Isentropic Process of the Ideal Gas
The isentropic process (a special case of adiabatic process) can be expressed with the ideal gas law as:
pVκ = constant
or
p1V1κ = p2V2κ
in which κ = cp/cv is the ratio of the specific heats (or heat capacities) for the gas. One for constant pressure (cp) and one for constant volume (cv). Note that, this ratio κ = cp/cv is a factor in determining the speed of sound in a gas and other adiabatic processes.
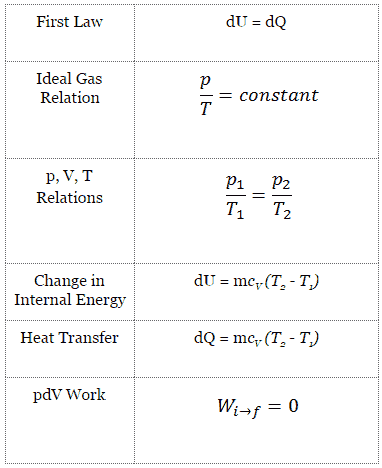
Isochoric Process
An isochoric process is a thermodynamic process, in which the volume of the closed system remains constant (V = const). It describes the behavior of gas inside the container, that cannot be deformed. Since the volume remains constant, the heat transfer into or out of the system does not the p∆V work, but only changes the internal energy (the temperature) of the system.
Isochoric Process and the First Law
The classical form of the first law of thermodynamics is the following equation:
dU = dQ – dW
In this equation dW is equal to dW = pdV and is known as the boundary work. Then:
dU = dQ – pdV
In isochoric process and the ideal gas, all of heat added to the system will be used to increase the internal energy.
Isochoric process (pdV = 0):
dU = dQ (for ideal gas)
dU = 0 = Q – W → W = Q (for ideal gas)
Isochoric Process of the Ideal Gas
The isochoric process can be expressed with the ideal gas law as:
or
On a p-V diagram, the process occurs along a horizontal line that has the equation V = constant.
See also: Guy-Lussac’s Law
Isobaric Process
An isobaric process is a thermodynamic process, in which the pressure of the system remains constant (p = const). The heat transfer into or out of the system does work, but also changes the internal energy of the system.
Since there are changes in internal energy (dU) and changes in system volume (∆V), engineers often use the enthalpy of the system, which is defined as:
H = U + pV
Isobaric Process and the First Law
The classical form of the first law of thermodynamics is the following equation:
dU = dQ – dW
In this equation dW is equal to dW = pdV and is known as the boundary work. In an isobaric process and the ideal gas, part of heat added to the system will be used to do work and part of heat added will increase the internal energy (increase the temperature). Therefore it is convenient to use the enthalpy instead of the internal energy.
Isobaric process (Vdp = 0):
dH = dQ → Q = H2– H1
At constant entropy, i.e. in isentropic process, the enthalpy change equals the flow process work done on or by the system.
Isobaric Process of the Ideal Gas
The isobaric process can be expressed with the ideal gas law as:
or
On a p-V diagram, the process occurs along a horizontal line (called an isobar) that has the equation p = constant.
See also: Charles’s Law
Diesel Cycle – pV, Ts diagram

Diesel cycles are often plotted on a pressure-volume diagram (pV diagram) and on a temperature-entropy diagram (Ts diagram).
When plotted on a pressure volume diagram, the isobaric process follow the isobar line for the gas (the horizontal lines), the isochoric process follow the isochoric line for the gas (the vertical line), adiabatic processes move between these lines and the area bounded by the complete cycle path represents the total work that can be done during one cycle.
The temperature-entropy diagram (Ts diagram) in which the thermodynamic state is specified by a point on a graph with specific entropy (s) as the horizontal axis and absolute temperature (T) as the vertical axis. Ts diagrams are a useful and common tool, particularly because it helps to visualize the heat transfer during a process. For reversible (ideal) processes, the area under the T-s curve of a process is the heat transferred to the system during that process.
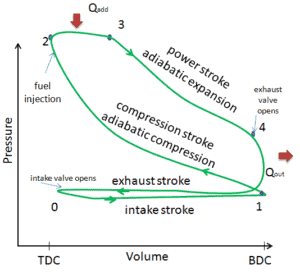
Four Stroke Diesel Engine
Diesel engines may be designed as either two stroke or four stroke cycles.The four stroke Diesel engine is an internal combustion (IC) engine in which the piston completes four separate strokes while turning a crankshaft. A stroke refers to the full travel of the piston along the cylinder, in either direction. Therefore each stroke does not correspond to single thermodynamic process given in chapter Diesel Cycle – Processes.
The four stroke engine comprises:
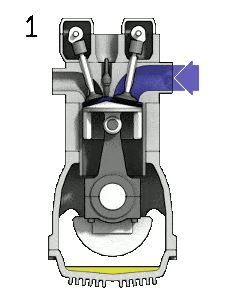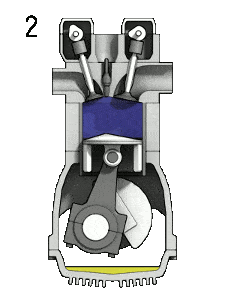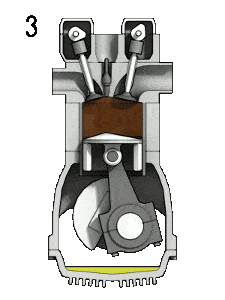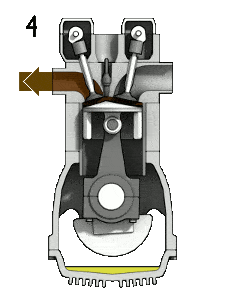
The Diesel engine is similar in operation to the gasoline engine. In this picture, there is an Otto engine, which is ignited by a spark plug instead of compression itself. Four stroke engine – Otto engine
Source: wikipedia.org, Own work of Zephyris, CC BY-SA 3.0the intake stroke – The piston moves from top dead center (TDC) to bottom dead center (BDC) and the cycle passes points 0 → 1. In this stroke the intake valve is open while the piston pulls air (without a fuel) into the cylinder by producing vacuum pressure into the cylinder through its downward motion.
- the compression stroke – The piston moves from bottom dead center (BDC) to top dead center (TDC) and the cycle passes points 1 → 2 . In this stroke both the intake and exhaust valves are closed, resulting in adiabatic air compression (i.e. without heat transfer to or from the environment). During this compression, the volume is reduced, the pressure and temperature both rise. At the end of this stroke fuel is injected and burns in the compressed hot air. At the end of this stroke the crankshaft has completed a full 360 degree revolution.
- the power stroke – The piston moves from top dead center (TDC) to bottom dead center (BDC) and the cycle passes points 2 → 3 → 4. In this stroke both the intake and exhaust valves are closed. At the beginning of the power stroke, a near isobaric combustion occur between 2 and 3. In this interval the pressure remains constant since the piston descends, and the volume increases. At 3 fuel injection and combustion are complete, and the cylinder contains gas at a higher temperature than at 2. Between 3 and 4 this hot gas expands, again approximately adiabatically. In this stroke the piston is driven towards the crankshaft, the volume in increased, and the work is done by the gas on the piston.
- the exhaust stroke. The piston moves from bottom dead center (BDC) to top dead center (TDC) and the cycle passes points 4 → 1 → 0. In this stroke the exhaust valve is open while the piston pulls an exhaust gases out of the chamber. At the end of this stroke the crankshaft has completed a second full 360 degree revolution.
Note that: In an ideal case, the adiabatic expansion should continue, until the pressure falls to that of the surrounding air. This would increase the thermal efficiency of such engine, but this also causes the practical difficulties with the engine. Simply the engine would have to be much larger.
Comparison of Actual and Ideal Diesel Cycles
 In this article it is shown an ideal Diesel cycle in which there are a lot of assumptions differs from actual Diesel cycle. The main differences between the actual and ideal Diesel engine appear in the figure. In reality, the ideal cycle does not occur and there are many losses associated with each process. For an actual cycle, the shape of the pV diagram is similar to the ideal, but the area (work) enclosed by the pV diagram is always less than the ideal value. The ideal Diesel cycle is based on the following assumptions:
In this article it is shown an ideal Diesel cycle in which there are a lot of assumptions differs from actual Diesel cycle. The main differences between the actual and ideal Diesel engine appear in the figure. In reality, the ideal cycle does not occur and there are many losses associated with each process. For an actual cycle, the shape of the pV diagram is similar to the ideal, but the area (work) enclosed by the pV diagram is always less than the ideal value. The ideal Diesel cycle is based on the following assumptions:
- Closed cycle: The largest difference between the two diagrams is the simplification of the intake and exhaust strokes in the ideal cycle. In the exhaust stroke, heat Qout is ejected to the environment (in a real engine, the gas leaves the engine and is replaced by a new mixture of air and fuel).
- Isobaric heat addition. In real engines the heat addition is never isobaric.
- No heat transfer
- Compression – The gas is compressed adiabatically from state 1 to state 2. In real engines, there are always some inefficiencies that reduce the thermal efficiency.
- Expansion. The gas expands adiabatically from state 3 to state 4.
- Complete combustion of the mixture.
- No pumping work. Pumping work is the difference between the work done during exhaust stroke and the work done during intake stroke. In real cycles, there is a pressure difference between exhaust and inlet pressures.
- No blowdown loss. Blowdown loss is caused by the early opening of exhaust valves. This results in a loss of work output during expansion stroke.
- No blow-by loss. The blow-by loss is caused by the leakage of compressed gases through piston rings and other crevices.
- No frictional losses.
These simplifying assumptions and losses lead to the fact that the enclosed area (work) of the pV diagram for an actual engine is significantly smaller than the size of the area (work) enclosed by the pV diagram of the ideal cycle. In other words, the ideal engine cycle will overestimate the net work and, if the engines run at the same speed, greater power produced by the actual engine by around 20% (similarly as in the case of Otto engine).
Compression Ratio – Otto Engine
The compression ratio, CR, is defined as the ratio of the volume at bottom dead center and the volume at top dead center. It is a key characteristics for many internal combustion engines. In the following section, it will be shown that the compression ratio determines the thermal efficiency of used thermodynamic cycle of the combustion engine. In general, it is desired to have a high compression ratio, because it allows an engine to reach higher thermal efficiency.
For example, let assume an Otto cycle with compression ratio of CR = 10 : 1. The volume of the chamber is 500 cm³ = 500×10-6 m3 (0.5l) prior to the compression stroke. For this engine all required volumes are known:
- V1 = V4 = Vmax = 500×10-6 m3 (0.5l)
- V2 = V3 = Vmin = Vmax / CR = 55.56 ×10-6 m3
Note that (Vmax – Vmin) x number of cylinders = total engine displacement.
Examples of Compression Ratios – Gasoline vs. Diesel
- The compression ratio in a gasoline-powered engine will usually not be much higher than 10:1 due to potential engine knocking (autoignition) and not lower than 6:1.
- A turbocharged Subaru Impreza WRX has a compression ratio of 8.0:1. In general, a turbocharged or supercharged engines already have compressed air at air intake, therefore they are usually built with lower compression ratio.
- A stock Honda S2000 engine (F22C1) has a compression ratio of 11.1:1.
- Some atmospheric sportscar engines can have compression ratio up to 12.5 : 1 (e.g. Ferrari 458 Italia).
- In 2012, Mazda released new petrol engines under the brand name SkyActiv with a 14:1 compression ratio. To reduce the risk of engine knocking, residual gas is reduced by using 4-2-1 engine exhaust systems, implementing a piston cavity, and optimizing fuel injection.
- The Diesel engines have the compression ratio that normally exceed 14:1 and ratios over 22:1 are also common.
Thermal Efficiency for Diesel Cycle
In general the thermal efficiency, ηth, of any heat engine is defined as the ratio of the work it does, W, to the heat input at the high temperature, QH.
The thermal efficiency, ηth, represents the fraction of heat, QH, that is converted to work. Since energy is conserved according to the first law of thermodynamics and energy cannot be be converted to work completely, the heat input, QH, must equal the work done, W, plus the heat that must be dissipated as waste heat QC into the environment. Therefore we can rewrite the formula for thermal efficiency as:
The heat absorbed occurs during combustion of fuel-air mixture, when the spark occurs, roughly at constant volume. Since during an isochoric process there is no work done by or on the system, the first law of thermodynamics dictates ∆U = ∆Q. Therefore the heat added and rejected are given by:
Qadd = mcp (T3 – T2)
Qout = mcv (T4 – T1)
Substituting these expressions for the heat added and rejected in the expression for thermal efficiency yields:
This equation can be rearranged to the form with the compression ratio and the cut-off ratio:
where
- ηDiesel is the maximum thermal efficiency of a Diesel cycle
- α is the cut-off ration V3/V2 (i.e. the ratio of volumes at the end and start of the combustion phase)
- CR is the compression ratio
- κ = cp/cv = 1.4
It is very useful conclusion, because it is desirable to achieve a high compression ratio to extract more mechanical energy from a given mass of the fuel. As we have concluded in previous section the air-standard Otto cycle thermal efficiency is also a function of compression ratio and κ.
When we compare these to formulae, it can be seen that for a given compression ratio (CR), the Otto cycle will be more efficient than the Diesel cycle . But diesel engines are usually be more efficient since they are able to operate at higher compression ratios.
In ordinary Otto engines the compression ratio has its limits. The compression ratio in a gasoline-powered engine will usually not be much higher than 10:1. Higher compression ratios will make gasoline engines subject to engine knocking, caused by autoignition an unburned mixture, if lower octane-rated fuel is used. In diesel engines there is minimal risk of autoignition of the fuel, because diesel engines are compression-ignition engines and there is no fuel in the cylinder at the beginning of the compression stroke.
Mean Effective Pressure – MEP
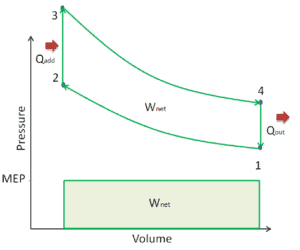
A parameter used by engineers to describe the performance of reciprocating piston engines is known as the mean effective pressure, or MEP. MEP is a very useful measure of an engine’s capacity to do work that is independent of engine displacement. There are several types of MEP. These MEPs are defined by the location measurement and method of calculation (e.g. BMEP or IMEP).
In general, the mean effective pressure is the theoretical constant pressure that, if it acted on the piston during the power stroke, would produce the same net work as actually developed in one complete cycle. The MEP can be defined as:
For example, the net indicated mean effective pressure, known as IMEPn is equal to the mean effective pressure calculated from in-cylinder pressure (there must be this measurement) over the complete engine cycle. Note that, it is 720° for a four-stroke engine and 360° for a two-stroke engine.
Some examples:
- MEP of an atmospheric gasoline engine can range from 8 to 11 bar in the region of maximum torque.
- MEP of a turbocharged gasoline engine can range from 12 to 17 bar.
- MEP of an atmospheric diesel engine can range from 7 to 9 bar.
- MEP of a turbocharged diesel engine can range from 14 to 18 bar
For example, a four-stroke gasoline engine producing 200 N·m from 2 litres of displacement has a MEP of (4π)(200 N·m)/(0.002 m³) = 1256000 Pa = 12 bar. As can be seen, the MEP is useful characteristics of an engine. For two engines of equal displacement volume, the one with a higher MEP would produce the greater net work and, if the engines run at the same speed, greater power.
Diesel Cycle – Problem with Solution

Let assume the Diesel cycle, which is the one of most common thermodynamic cycles that can be found in automobile engines. One of key parameters of such engines is the change in volumes between top dead center (TDC) to bottom dead center (BDC). The ratio of these volumes (V1 / V2) is known as the compression ratio. Also the cut-off ratio V3/V2, which is the ratio of volumes at the end and start of the combustion phase.
In this example let assume the Diesel cycle with compression ratio of CR = 20 : 1 and cut-off ratio α = 2. The air is at 100 kPa = 1 bar, 20 °C (293 K), and the volume of the chamber is 500 cm³ prior to the compression stroke.
- Specific heat capacity at constant pressure of air at atmospheric pressure and room temperature: cp = 1.01 kJ/kgK.
- Specific heat capacity at constant volume of air at atmospheric pressure and room temperature: cv = 0.718 kJ/kgK.
- κ = cp/cv = 1.4
Calculate:
- the mass of intake air
- the temperature T2
- the pressure p2
- the temperature T3
- the amount of heat added by burning of fuel-air mixture
- the thermal efficiency of this cycle
- the MEP
Solution:
1)
At the beginning of calculations we have to determine the amount of gas in the cylinder before the compression stroke. Using the ideal gas law, we can find the mass:
pV = mRspecificT
where:
- p is the absolute pressure of the gas
- m is the mass of substance
- T is the absolute temperature
- V is the volume
- Rspecific is the specific gas constant, equal to the universal gas constant divided by the molar mass (M) of the gas or mixture. For dry air Rspecific = 287.1 J.kg-1.K-1.
Therefore
m = p1V1/RspecificT1 = (100000 × 500×10-6 )/(287.1 × 293) = 5.95×10-4 kg
2)
In this problem all volumes are known:
- V1 = V4 = Vmax = 500×10-6 m3 (0.5l)
- V2 = Vmin = Vmax / CR = 25 ×10-6 m3
Note that (Vmax – Vmin) x number of cylinders = total engine displacement
Since the process is adiabatic, we can use the following p, V, T relation for adiabatic processes:
thus
T2 = T1 . CRκ – 1 = 293 . 200.4 = 971 K
3)
Again, we can use the ideal gas law to find the pressure at the end of the compression stroke as:
p2 = mRspecificT2 / V2 = 5.95×10-4 x 287.1 x 971 / 25 ×10-6 = 6635000 Pa = 66.35 bar
4)
Since process 2 → 3 occurs at constant pressure, the ideal gas equation of state gives
T3 = (V3/V2) x T2 = 1942 K
To calculate the amount of heat added by burning of fuel-air mixture, Qadd, we have to use the first law of thermodynamics for isobaric process, which states:
Qadd = mcp (T3 – T2) = 5.95×10-4 x 1010 x 971 = 583.5 J
5)
Thermal efficiency for this Diesel cycle:
As was derived in the previous section, the thermal efficiency of Diesel cycle is a function of the compression ratio, the cut-off ratio and κ:
- ηDiesel is the maximum thermal efficiency of a Diesel cycle
- α is the cut-off ration V3/V2 (i.e. the ratio of volumes at the end and start of the combustion phase)
- CR is the compression ratio
- κ = cp/cv = 1.4
For this example:
ηDiesel = 0.6467 = 64.7%
6)
The MEP was defined as:
It this equation the displacement volume is equal to Vmax – Vmin. The net work for one cycle can be calculated using the heat added and the thermal efficiency:
Wnet = Qadd . ηOtto = 583.5 x 0.6467 = 377.3 J
MEP = 377.3 / (500×10-6 – 25 ×10-6) = 794.3 kPa = 7.943 bar
We hope, this article, Diesel Cycle – Diesel Engine, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.