Gain insights into the Joule-Thomson Effect, a crucial thermodynamic process for understanding refrigeration and gas liquefaction.
Understanding the Joule-Thomson Effect
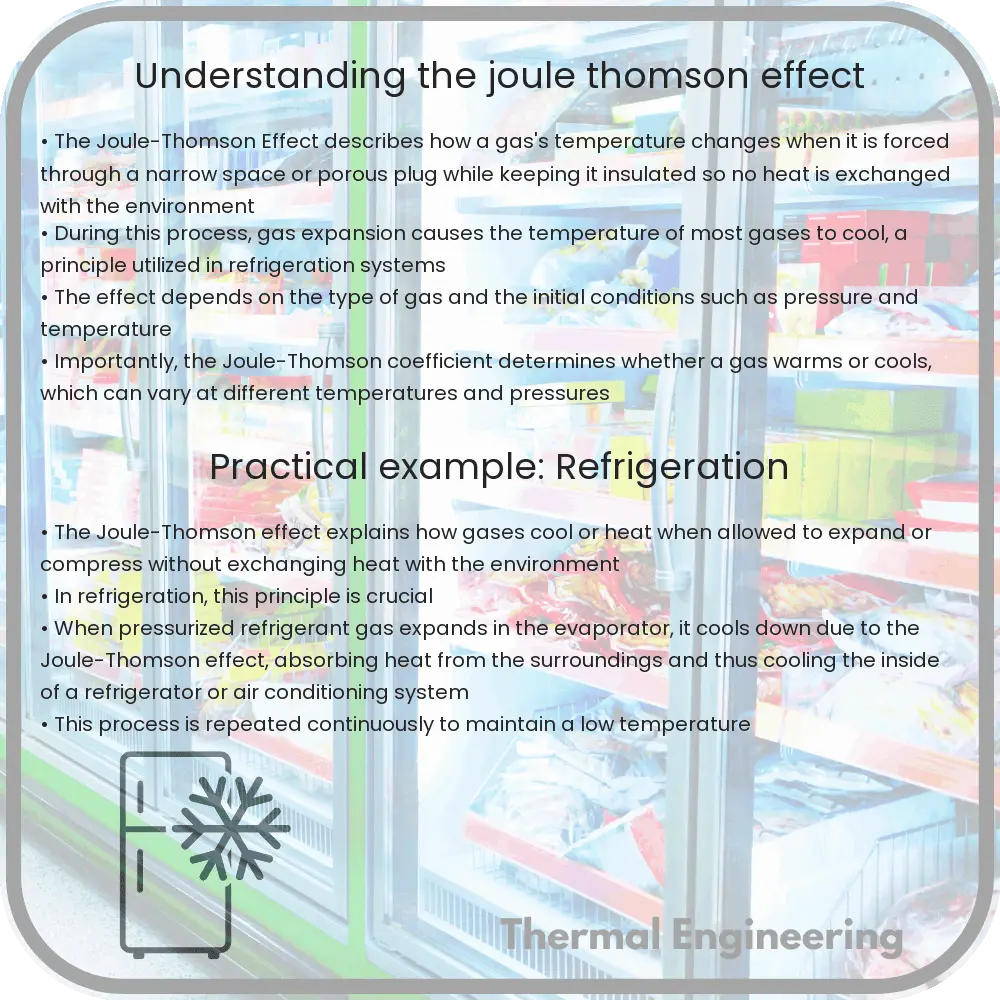
The Joule-Thomson effect, also known as the Joule-Kelvin effect, is a thermodynamic process that describes the temperature change of a real gas when it is forced through a valve or porous plug while keeping it insulated so that no heat is exchanged with the environment. This phenomenon is crucial for understanding how refrigeration cycles, such as those used in air conditioners and natural gas processing, work.
Principles Behind the Joule-Thomson Effect
At the heart of the Joule-Thomson effect is the idea that real gases do not always behave like ideal gases. The behavior of real gases can deviate significantly from the predictions by the ideal gas law, particularly under conditions of high pressure and low temperature. The phenomenon involves various intermolecular forces present in real gases.
When a gas expands, its particles have more room to move and spread out, which means they use some of their internal energy to overcome intermolecular forces. Thus, if the expansion is isenthalpic (occurs at constant enthalpy), and no external work is done (e.g., the gas does neither work on the surroundings nor is work done on it), and there’s no heat exchange (adiabatic process), the internal energy used in overcoming intermolecular attractions can only come from the internal energy stored in the temperature of the gas, causing it to cool.
Mathematical Description of the Effect
The temperature change in the Joule-Thomson effect is quantified by the Joule-Thomson coefficient (\(\mu\)), which can be defined as:
\[\mu = \left(\frac{\partial T}{\partial P}\right)_H\]
Where \(\partial T/\partial P\) is the rate of change of temperature \(T\) with respect to pressure \(P\) at constant enthalpy \(H\). The sign and magnitude of \(\mu\) indicate how the temperature will change at a given pressure.
- If \(\mu > 0\), the gas cools upon expansion (positive Joule-Thomson coefficient).
- If \(\mu < 0\), the gas heats up during expansion (negative Joule-Thomson coefficient).
The inversion temperature is a unique property of each gas and determines whether the Joule-Thomson effect will result in heating or cooling. It represents the temperature above which the Joule-Thomson coefficient becomes negative (the gas warms when expanded) and below which it becomes positive (the gas cools when expanded).
Applications of the Joule-Thomson Effect
The Joule-Thomson effect has practical applications in various industries, especially those involving the liquefaction of gases, refrigeration, and HVAC (heating, ventilation, and air conditioning) systems.
- Gas Liquefaction: The cooling effect of the Joule-Thomson expansion is employed in the liquefaction of gases such as nitrogen, oxygen, and natural gas. These gases can then be transported more efficiently in a liquid state.
- Refrigeration Cycles: Many refrigeration systems operate based on the Joule-Thomson effect. When compressed gas expands through a throttle valve, it cools down and is used to cool an enclosed space.
- Heat Pumps and Air Conditioners: The reverse process is used in heat pumps and air conditioners, where the Joule-Thomson effect helps in heating environments while cooling the gas.
Understanding the Joule-Thomson effect not only helps in designing efficient thermal machines but also enhances our comprehension of thermodynamic processes and the nature of real gases.