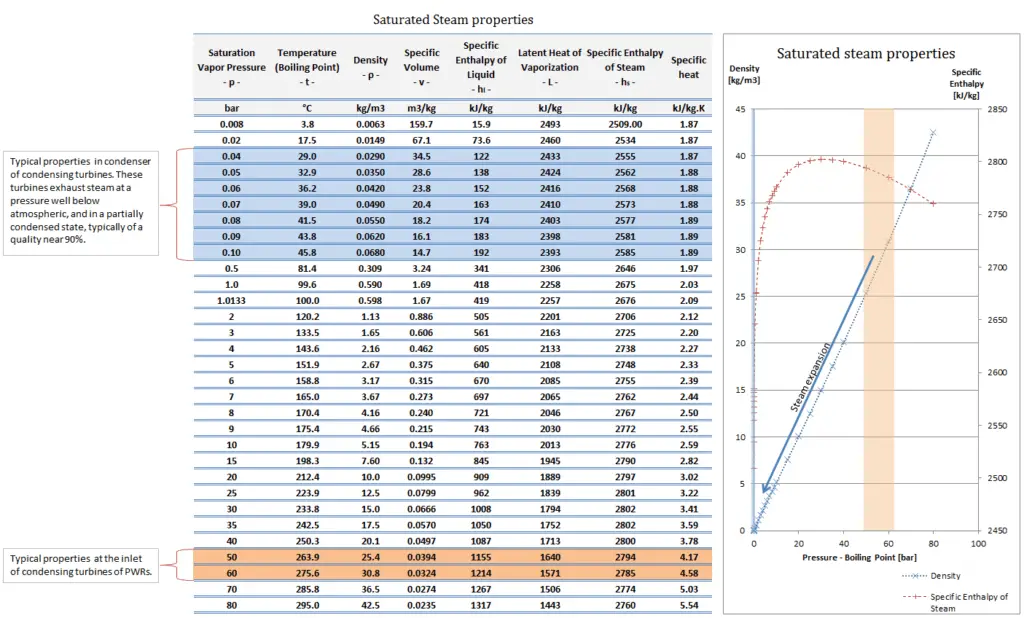
Density of Steam
 Water and steam are a common medium because their properties are very well known. Their properties are tabulated in so called “Steam Tables”. In these tables the basic and key properties, such as pressure, temperature, enthalpy, density and specific heat, are tabulated along the vapor-liquid saturation curve as a function of both temperature and pressure.
Water and steam are a common medium because their properties are very well known. Their properties are tabulated in so called “Steam Tables”. In these tables the basic and key properties, such as pressure, temperature, enthalpy, density and specific heat, are tabulated along the vapor-liquid saturation curve as a function of both temperature and pressure.
The density (⍴) of any substance is the reciprocal of its specific volume (ν).
ρ = m/V = 1/ν
The specific volume (ν) of a substance is the total volume (V) of that substance divided by the total mass (m) of that substance (volume per unit mass). It has units of cubic meter per kilogram (m3/kg).
Density of Supercritical Fluid
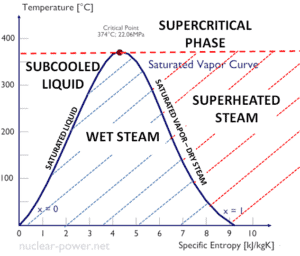
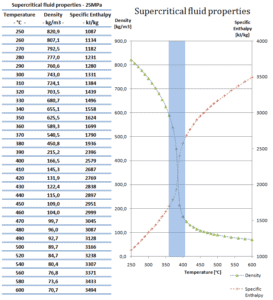 A supercritical fluid is a fluid that is at pressures higher than its thermodynamic critical values. At the critical and supercritical pressures a fluid is considered as a single-phase substance in spite of the fact that all thermophysical properties undergo significant changes within the critical and pseudocritical regions.
A supercritical fluid is a fluid that is at pressures higher than its thermodynamic critical values. At the critical and supercritical pressures a fluid is considered as a single-phase substance in spite of the fact that all thermophysical properties undergo significant changes within the critical and pseudocritical regions.
At pressures above the critical pressure, properties of water in the reactor change gradually and continuously from those we ordinarily associate with a liquid (high density, small compressibility) to those of a gas (low density, large compressibility) without a phase change. There is no change in the phase of water in the core. On the other hand, physical properties such as density, specific heat, specific enthalpy undergo significant changes, especially in the temperature range of the pseudocritical region (for 25 MPa between 372°C and 392°C).
We hope, this article, Density of Steam – Specific Volume of Steam, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.