Energy can neither be created nor destroyed
It is the principle of conservation of energy, meaning that:
Energy can neither be created nor destroyed, but rather transformed into various forms.
In thermodynamics, it is the most important law for analysis of most systems and the one that quantifies how thermal energy is transformed to other forms of energy. It follows, perpetual motion machines of the first kind are impossible.
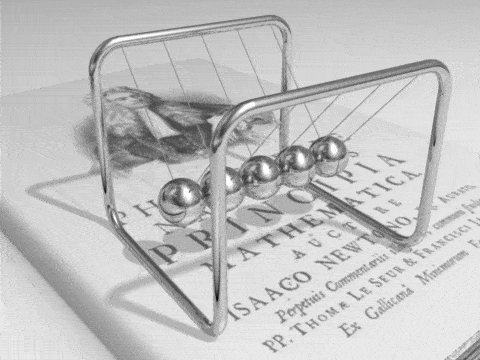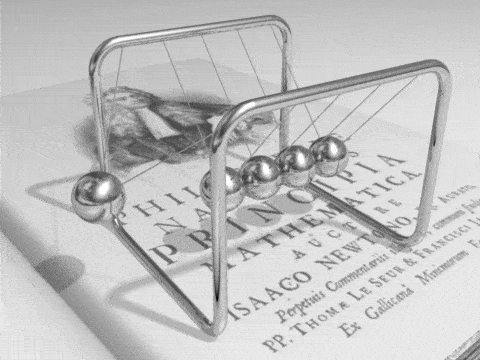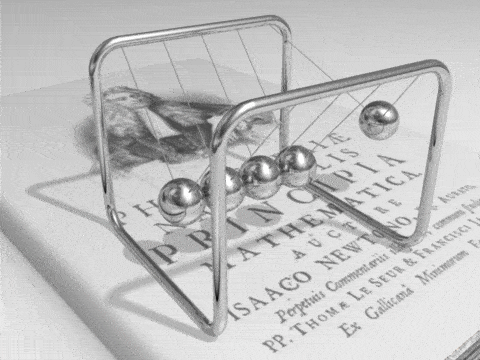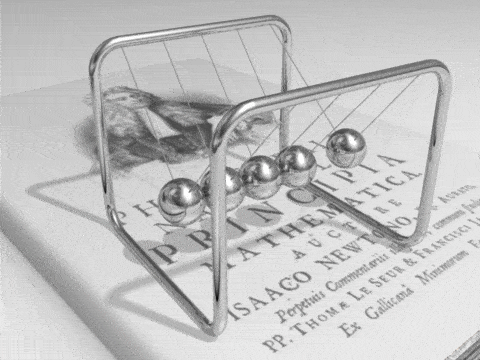
Energy can be defined as the capacity for doing work. It may exist in a variety of forms and may be transformed from one type of energy to another in hundreds of ways.
For example, burning gasoline to power cars is an energy conversion process we rely on. The chemical energy in gasoline is converted to thermal energy, which is then converted to mechanical energy that makes the car move. The mechanical energy has been converted to kinetic energy. When we use the brakes to stop a car, that kinetic energy is converted by friction back to heat, or thermal energy.
A consequence of the law of conservation of energy is that a perpetual motion machine of the first kind, which produces work without the input of energy, cannot exist.
The concept of energy conservation is widely used in many fields. In this article the following fields are discussed:
- Conservation of Mechanical Energy
- Conservation of Energy in Fluid Mechanics
- Conservation of Energy in Thermodynamics
- Conservation of Energy in Electrical Circuits
- Conservation of Energy in Chemical Reactions
- Conservation of Energy in Special Relativity Theory
- Conservation of Energy in Nuclear Reactions
Law of Conservation of Mass-Energy – Mass-Energy Equivalence
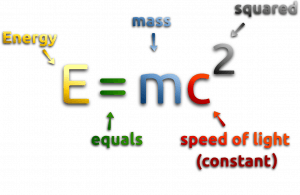 At the beginning of the 20th century, the notion of mass underwent a radical revision. Mass lost its absoluteness. One of the striking results of Einstein’s theory of relativity is that mass and energy are equivalent and convertible one into the other. Equivalence of the mass and energy is described by Einstein’s famous formula E = mc2. In words, energy equals mass multiplied by the speed of light squared. Because the speed of light is a very large number, the formula implies that any small amount of matter contains a very large amount of energy. The mass of an object was seen to be equivalent to energy, to be interconvertible with energy, and to increase significantly at exceedingly high speeds near that of light. The total energy of an object was understood to comprise its rest mass as well as its increase of mass caused by increase in kinetic energy.
At the beginning of the 20th century, the notion of mass underwent a radical revision. Mass lost its absoluteness. One of the striking results of Einstein’s theory of relativity is that mass and energy are equivalent and convertible one into the other. Equivalence of the mass and energy is described by Einstein’s famous formula E = mc2. In words, energy equals mass multiplied by the speed of light squared. Because the speed of light is a very large number, the formula implies that any small amount of matter contains a very large amount of energy. The mass of an object was seen to be equivalent to energy, to be interconvertible with energy, and to increase significantly at exceedingly high speeds near that of light. The total energy of an object was understood to comprise its rest mass as well as its increase of mass caused by increase in kinetic energy.
In special theory of relativity certain types of matter may be created or destroyed, but in all of these processes, the mass and energy associated with such matter remains unchanged in quantity. It was found the rest mass an atomic nucleus is measurably smaller than the sum of the rest masses of its constituent protons, neutrons and electrons. Mass was no longer considered unchangeable in the closed system. The difference is a measure of the nuclear binding energy which holds the nucleus together. According to the Einstein relationship (E = mc2) this binding energy is proportional to this mass difference and it is known as the mass defect.
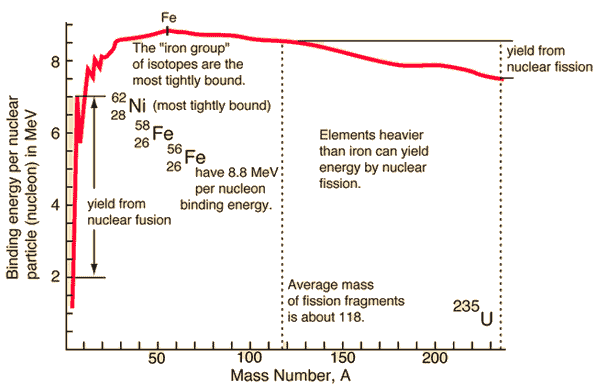
Source: hyperphysics.phy-astr.gsu.edu
During the nuclear splitting or nuclear fusion, some of the mass of the nucleus gets converted into huge amounts of energy and thus this mass is removed from the total mass of the original particles, and the mass is missing in the resulting nucleus. The nuclear binding energies are enormous, they are of the order of a million times greater than the electron binding energies of atoms.
Generally, in both chemical and nuclear reactions, some conversion between rest mass and energy occurs, so that the products generally have smaller or greater mass than the reactants. Therefore the new conservation principle is the conservation of mass-energy.
See also: Energy Release from Fission
We hope, this article, Energy can neither be created nor destroyed, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.
