Thermo-chemical Storage
One of three possible approaches to thermal energy storage is to use reversible thermo-chemical reactions. The most important advantage of the thermo-chemical storage method is that the enthalpy of reaction is considerably larger than the specific heat or the heat of fusion. Therefore the storage density is much better. In chemical reactions, energy is stored in the chemical bonds between the atoms that make up the molecules. Energy storage on the atomic level includes energy associated with electron orbital states. Whether a chemical reaction absorbs or releases energy, there is no overall change in the amount of energy during the reaction. That’s because of the law of conservation of energy, which states that:
Energy cannot be created or destroyed. Energy may change form during a chemical reaction.
One example of an experimental storage system based on chemical reaction energy is the salt hydrate technology. The system is especially advantageous for seasonal thermal energy storage. The system uses the reaction energy created when salts are hydrated or dehydrated. It works by storing heat in a container containing 50% sodium hydroxide (NaOH) solution. Heat (e.g. from using a solar collector) is stored by evaporating the water in an endothermic reaction. When water is added again, heat is released in an exothermic reaction at 50 °C. Current systems operate at 60% efficiency.
Thermal Energy Storage
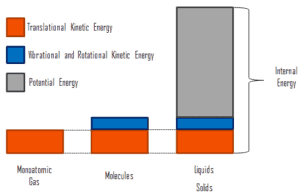 In thermodynamics, internal energy (also called the thermal energy) is defined as the energy associated with microscopic forms of energy. It is an extensive quantity, it depends on the size of the system, or on the amount of substance it contains. The SI unit of internal energy is the joule (J). It is the energy contained within the system, excluding the kinetic energy of motion of the system as a whole and the potential energy of the system. Microscopic forms of energy include those due to the rotation, vibration, translation, and interactions among the molecules of a substance. None of these forms of energy can be measured or evaluated directly, but techniques have been developed to evaluate the change in the total sum of all these microscopic forms of energy.
In thermodynamics, internal energy (also called the thermal energy) is defined as the energy associated with microscopic forms of energy. It is an extensive quantity, it depends on the size of the system, or on the amount of substance it contains. The SI unit of internal energy is the joule (J). It is the energy contained within the system, excluding the kinetic energy of motion of the system as a whole and the potential energy of the system. Microscopic forms of energy include those due to the rotation, vibration, translation, and interactions among the molecules of a substance. None of these forms of energy can be measured or evaluated directly, but techniques have been developed to evaluate the change in the total sum of all these microscopic forms of energy.
In addition, energy is can be stored in the chemical bonds between the atoms that make up the molecules. This energy storage on the atomic level includes energy associated with electron orbital states, nuclear spin, and binding forces in the nucleus.

Thermal energy can be also very effectively stored. Nowadays, situation on energy markets is different. The increasing on the prices of the conventional energy sources and the environmental awareness have leaded to increase the use of renewable energies and the energy efficiency. Thermal energy storage forms a key component of a power plant for improvement of its dispatchability, especially for concentrating solar power plants (CSP). Thermal energy storage (TES) is achieved with widely differing technologies. There are three methods used and still being investigated in order to store thermal energy.
- Sensible Heat Storage (SHS)
- Latent Heat Storage (LHS)
- Thermo-chemical Storage
We hope, this article, Thermo-chemical Storage, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.