Definition of Fluid
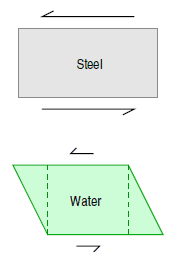
By definition, a solid material is rigid. For example, if one were to impose a shear stress on a solid block of steel, the block would not begin to change shape until an extreme amount of stress has been applied. To be more exact, when a shear stress is first applied to a rigid material it deforms slightly, but then springs back to its original shape when the stress is relieved.
Source: wikipedia.org CC BY-SA
A plastic material, such as clay, also possess some degree of rigidity. However, the critical shear stress above which it yields is relatively small, and once this stress is exceeded the material deforms continuously and irreversibly, and does not recover
its original shape when the stress is relieved.
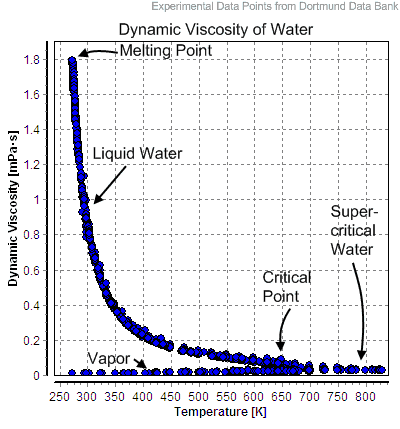
By definition, a fluid material possesses no rigidity at all. For example, if one were to impose a shear stress on a fluid element, the fluid element deforms, because it is unable to withstand any tendency of an applied shear stress to change its shape. Furthermore, the more stress that is applied, the more the fluid element will deform. This provides us with a characterizing feature of liquids (and gases—fluids, in general) that distinguishes them from other forms of matter, and we can thus give a formal definition.
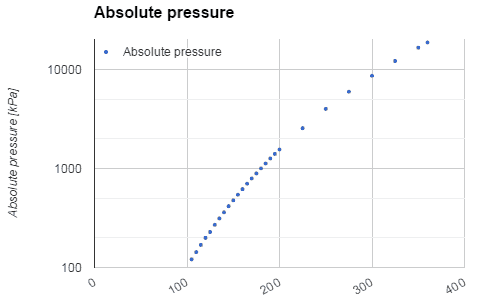
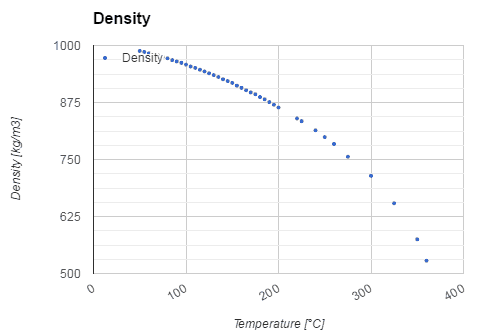
There are two types of fluid: liquids and gases. The most important difference between these two types of fluid is in their relative compressibility. Gases can be compressed much more easily than liquids. Consequently, any motion that involves significant pressure variations is generally accompanied by much larger changes in mass density in the case of a gas than in the case of a liquid.
Water as a reactor coolant
Water and steam are a common fluid used for heat exchange in the primary circuit (from surface of fuel rods to the coolant flow) and in the secondary circuit. It used due to its availability and high heat capacity, both for cooling and heating. It is especially effective to transport heat through vaporization and condensation of water because of its very large latent heat of vaporization.
A disadvantage is that water moderated reactors have to use high pressure primary circuit in order to keep water in liquid state and in order to achieve sufficient thermodynamic efficiency. Water and steam also reacts with metals commonly found in industries such as steel and copper that are oxidized faster by untreated water and steam. In almost all thermal power stations (coal, gas, nuclear), water is used as the working fluid (used in a closed loop between boiler, steam turbine and condenser), and the coolant (used to exchange the waste heat to a water body or carry it away by evaporation in a cooling tower).
Water as a moderator
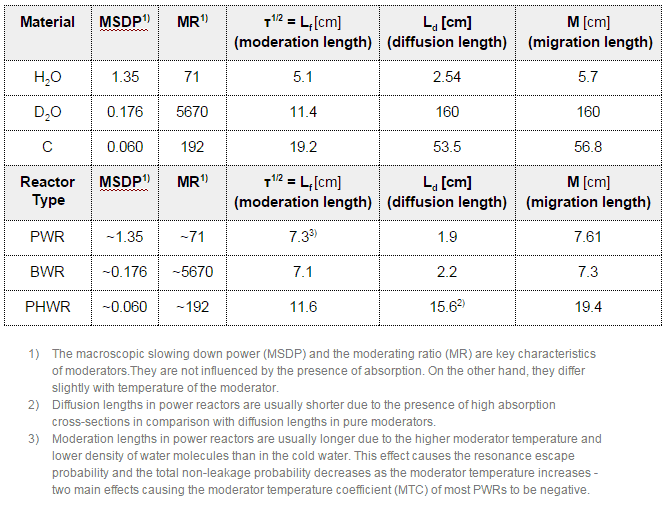
The neutron moderator, which is of importance in thermal reactors, is used to moderate, that is to slow down neutrons from fission to thermal energies. Nuclei with low mass numbers are most effective for this purpose, so the moderator is always a low-mass-number material. Commonly used moderators include regular (light) water (roughly 75% of the world’s reactors), solid graphite (20% of reactors) and heavy water (5% of reactors).
In most nuclear reactors, water is both a coolant and a moderator. The moderation occurs especially on hydrogen nuclei. In case of the hydrogen (A = 1) as the target nucleus, the incident neutron can be completely stopped – it has the highest average logarithmic energy decrement of all nuclei. On the other hand hydrogen nuclei have relatively higher absorption cross section, therefore water is not the best moderator according to the moderating ratio.
Water as a neutron shielding
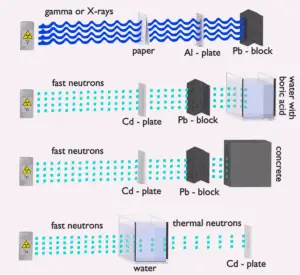
Water due to the high hydrogen content and the availability is efective and common neutron shielding. However, due to the low atomic number of hydrogen and oxygen, water is not acceptable shield against the gamma rays. On the other hand in some cases this disadvantage (low density) can be compensated by high thickness of the water shield. In case of neutrons, water perfectly moderates neutrons, but with absorption of neutrons by hydrogen nucleus secondary gamma rays with the high energy are produced. These gamma rays highly penetrates matter and therefore it can increase requirements on the thickness of the water shield. Adding a boric acid can help with this problem (neutron absorbtion on boron nuclei without strong gamma emission), but results in another problems with corrosion of construction materials.
See also: Shielding of Neutrons
We hope, this article, Definition of Fluid, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.