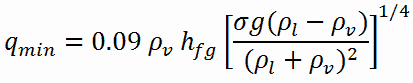Transition Boiling – Partial Film Boiling
Categorization by the wall superheat temperature, ΔTsat:
 The pioneering work on boiling was done in 1934 by S. Nukiyama, who used electrically heated nichrome and platinum wires immersed in liquids in his experiments. Nukiyama was the first to identify different regimes of pool boiling using his apparatus. He noticed that boiling takes different forms, depending on the value of the wall superheat temperature ΔTsat (known also as the excess temperature), which is defined as the difference between the wall temperature, Twall and the saturation temperature, Tsat.
The pioneering work on boiling was done in 1934 by S. Nukiyama, who used electrically heated nichrome and platinum wires immersed in liquids in his experiments. Nukiyama was the first to identify different regimes of pool boiling using his apparatus. He noticed that boiling takes different forms, depending on the value of the wall superheat temperature ΔTsat (known also as the excess temperature), which is defined as the difference between the wall temperature, Twall and the saturation temperature, Tsat.
Four different boiling regimes of pool boiling (based on the excess temperature) are observed:
- Natural Convection Boiling ΔTsat < 5°C
- Nucleate Boiling 5°C < ΔTsat < 30°C
- Transition Boiling 30°C < ΔTsat < 200°C
- Film Boiling 200°C < ΔTsat
Transition Boiling
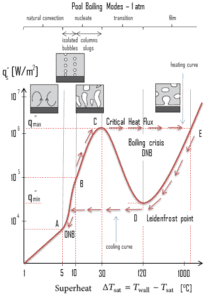
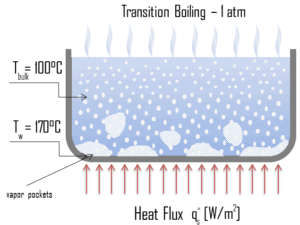 The nucleate boiling heat flux cannot be increased indefinitely. At some value, we call it the “critical heat flux” (CHF), the steam produced can form an insulating layer over the surface, which in turn deteriorates the heat transfer coefficient. This is because a large fraction of the surface is covered by a vapor film, which acts as an thermal insulation due to the low thermal conductivity of the vapor relative to that of the liquid. Immediately after the critical heat flux has been reached, boiling become unstable and transition boiling occurs. The transition from nucleate boiling to film boiling is known as the “boiling crisis”. Since beyond the CHF point the heat transfer coefficient decreases, the transition to film boiling is usually inevitable.
The nucleate boiling heat flux cannot be increased indefinitely. At some value, we call it the “critical heat flux” (CHF), the steam produced can form an insulating layer over the surface, which in turn deteriorates the heat transfer coefficient. This is because a large fraction of the surface is covered by a vapor film, which acts as an thermal insulation due to the low thermal conductivity of the vapor relative to that of the liquid. Immediately after the critical heat flux has been reached, boiling become unstable and transition boiling occurs. The transition from nucleate boiling to film boiling is known as the “boiling crisis”. Since beyond the CHF point the heat transfer coefficient decreases, the transition to film boiling is usually inevitable.
Critical Heat Flux
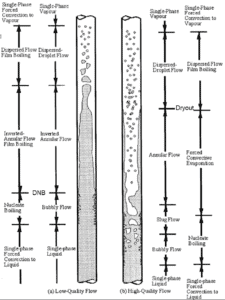 As was written, in nuclear reactors, limitations of the local heat flux is of the highest importance for reactor safety. For pressurized water reactors and also for boiling water reactors, there are thermal-hydraulic phenomena, which cause a sudden decrease in the efficiency of heat transfer (more precisely in the heat transfer coefficient). These phenomena occur at certain value of heat flux, known as the “critical heat flux”. The phenomena, that cause the deterioration of heat transfer are different for PWRs and for BWRs.
As was written, in nuclear reactors, limitations of the local heat flux is of the highest importance for reactor safety. For pressurized water reactors and also for boiling water reactors, there are thermal-hydraulic phenomena, which cause a sudden decrease in the efficiency of heat transfer (more precisely in the heat transfer coefficient). These phenomena occur at certain value of heat flux, known as the “critical heat flux”. The phenomena, that cause the deterioration of heat transfer are different for PWRs and for BWRs.
In both types of reactors, the problem is more or less associated with departure from nucleate boiling. The nucleate boiling heat flux cannot be increased indefinitely. At some value, we call it the “critical heat flux” (CHF), the steam produced can form an insulating layer over the surface, which in turn deteriorates the heat transfer coefficient. Immediately after the critical heat flux has been reached, boiling become unstable and film boiling occurs. The transition from nucleate boiling to film boiling is known as the “boiling crisis”. As was written, the phenomena, that cause the deterioration of heat transfer are different for PWRs and for BWRs.
Minimum Heat Flux – Leidenfrost Point
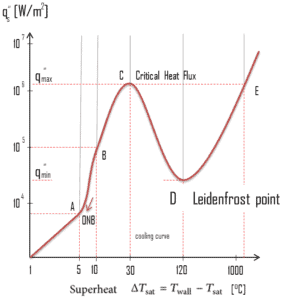 The Leidenfrost point, which corresponds to the minimal heat flux, is of practical interest since it represents the lower limit for the heat flux in the film boiling regime. If the heat flux drops below this minimum, the film will collapse, causing the surface to cool and nucleate boiling to be reestablished. Therefore, at this point, return to nucleate boiling (RNB) occurs. The terms quenching, minimum heat flux, return to nucleate boiling, departure from film boiling, film boiling collapse, and Leidenfrost point have been used interchangeably to refer to various forms of rewetting, but they are not exactly synonymous.
The Leidenfrost point, which corresponds to the minimal heat flux, is of practical interest since it represents the lower limit for the heat flux in the film boiling regime. If the heat flux drops below this minimum, the film will collapse, causing the surface to cool and nucleate boiling to be reestablished. Therefore, at this point, return to nucleate boiling (RNB) occurs. The terms quenching, minimum heat flux, return to nucleate boiling, departure from film boiling, film boiling collapse, and Leidenfrost point have been used interchangeably to refer to various forms of rewetting, but they are not exactly synonymous.
Using the stability theory, Zuber derived the following expression for the minimum heat flux (and corresponding Leidenfrost point) for a large horizontal plate:
where
- qmin – minimal heat flux [W/m2]
- hfg – enthalpy of vaporization, J/kg
- g – gravitational acceleration m/s2
- ρl — density of the liquid kg/m3
- ρv — density of vapour kg/m3
- σ — surface tension-liquid-vapour interface N/m
Leidenfrost Effect
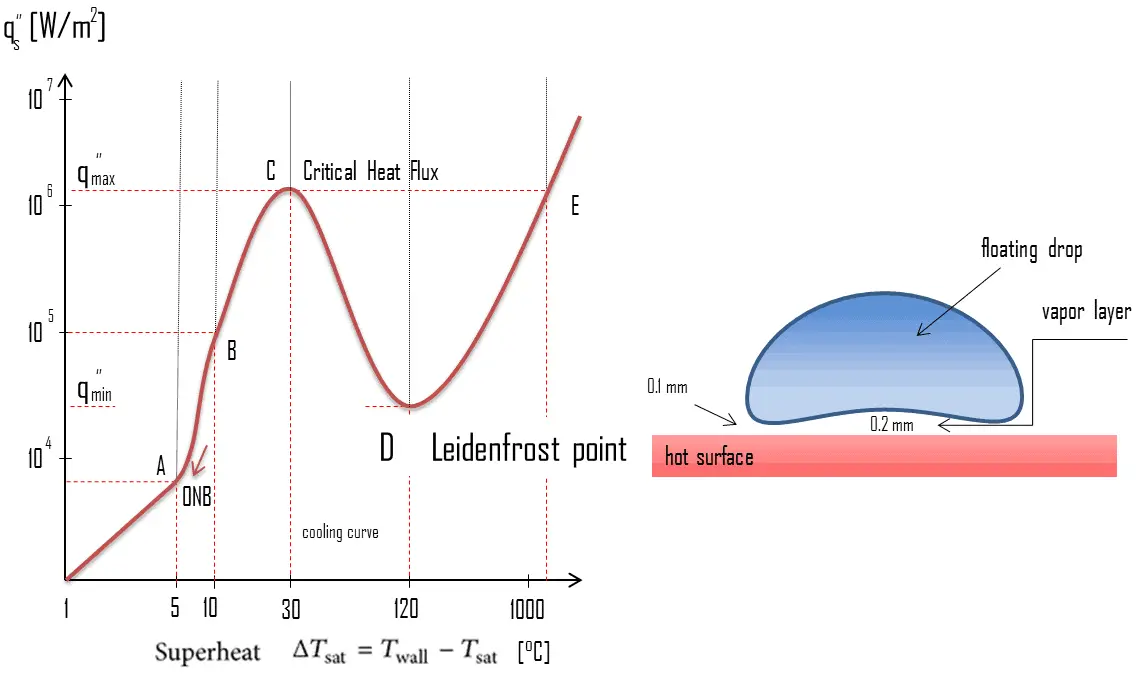
The Leidenfrost effect is a physical phenomenon in which a liquid, in near contact with a mass significantly hotter (e.g. a water drop in a hot pan) than the liquid’s boiling point, produces an insulating vapor layer keeping that liquid from boiling rapidly. The fact that a water drop is long lived when deposited on metal that is much hotter than the boiling temperature of water was first reported by Hermann Boerhaave in 1732. It was not investigated extensively until 1756 when a German doctor Johann Gottlob Leidenfrost published ‘‘A Tract About Some Qualities of Common Water.’’
This effect is can be commonly demonstrated during cooking when one sprinkles drops of water in a pan to gauge its temperature: if the pan’s temperature is at or above the Leidenfrost point, the water skitters across the pan and takes longer to evaporate than in a pan below the temperature of the Leidenfrost point (but still above boiling temperature). The Leidenfrost point, which corresponds to the minimal heat flux, is of practical interest since it represents the lower limit for the heat flux in the film boiling regime. If the heat flux drops below this minimum, the film will collapse, causing the surface to cool and nucleate boiling to be reestablished. The Leidenfrost effect is also responsible for the ability of liquid nitrogen to skitter across floors.
We hope, this article, Transition Boiling – Partial Film Boiling, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.