Energy Units
Energy is generally defined as the potential to do work or produce heat. This definition causes the SI unit for energy is the same as the unit of work – the joule (J). Joule is a derived unit of energy and it is named in honor of James Prescott Joule and his experiments on the mechanical equivalent of heat. In more fundamental terms, 1 joule is equal to:
1 J = 1 kg.m2/s2
Since energy is a fundamental physical quantity and it is used in various physical and engineering branches, there are many energy units in physics and engineering.
Calorie (unit cal) – Energy Unit
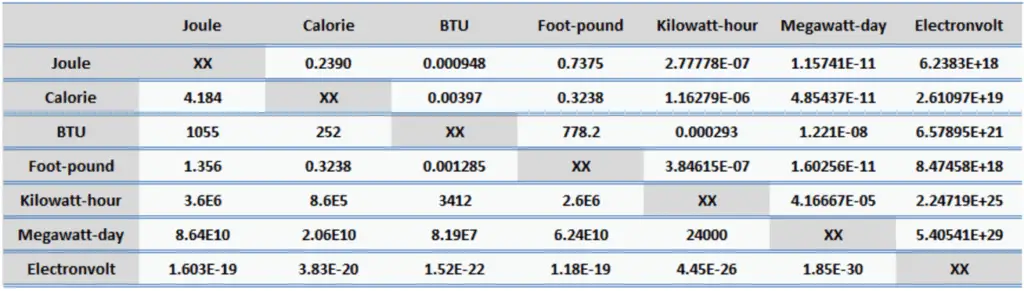
Calorie (unit: cal). Calorie is a traditional unit of heat. It is part of the International System of Units (SI). It is defined to be the amount of heat that must be absorbed by 1 gram of water to raise its temperature by 1 °C. Its counterpart in the British Imperial system of units is the BTU, which is defined as the amount of heat required to raise the temperature of one pound of water by one degree Fahrenheit. But we have to distinguish between small calorie and large calorie. The large calorie (unit: Cal) is defined in terms of the kilogram rather than the gram. It is equal to 1000 small calories and it is i.e. 1 kilocalorie (unit: kcal). It is used by nutritionists to characterize the energy-producing potential in food.
- 1 calorie = 4.184 J
- 1 calorie = 0.00397 BTU
- 1 calorie = 1.16 x 10-6 kWh
We hope, this article, Calorie (unit cal) – Energy Unit, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.