Pressure Formula – Pressure Equation
 Pressure is a measure of the force exerted per unit area on the boundaries of a substance. The standard unit for pressure in the SI system is the Newton per square meter or pascal (Pa). Mathematically (pressure formula):
Pressure is a measure of the force exerted per unit area on the boundaries of a substance. The standard unit for pressure in the SI system is the Newton per square meter or pascal (Pa). Mathematically (pressure formula):
p = F/A
where
- p is the pressure
- F is the normal force
- A is the area of the boundary
Pascal is defined as force of 1N that is exerted on unit area.
- 1 Pascal = 1 N/m2
- 1 MPa 106 N/m2
- 1 bar 105 N/m2
- 1 kPa 103 N/m2
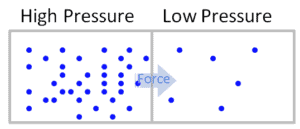
In general, pressure or the force exerted per unit area on the boundaries of a substance is caused by the collisions of the molecules of the substance with the boundaries of the system. As molecules hit the walls, they exert forces that try to push the walls outward. The forces resulting from all of these collisions cause the pressure exerted by a system on its surroundings. Pressure as an intensive variable is constant in a closed system. It really is only relevant in liquid or gaseous systems.
Ideal Gas Law
Any equation that relates the pressure, temperature, and specific volume of a substance is called an equation of state. The simplest and best-known equation of state for substances in the gas phase is the Ideal Gas equation of state. It was first stated by Émile Clapeyron in 1834 as a combination of the empirical Boyle’s law, Charles’ law and Avogadro’s Law. This equation predicts the p-v-T behavior of a gas quite accurately for dilute or low-pressure gases. In an ideal gas, molecules have no volume and do not interact. According to the ideal gas law, pressure varies linearly with temperature and quantity, and inversely with volume.
pV = nRT
where:
- p is the absolute pressure of the gas
- n is the amount of substance
- T is the absolute temperature
- V is the volume
- R is the ideal, or universal, gas constant, equal to the product of the Boltzmann constant and the Avogadro constant,
In this equation the symbol R is a constant called the universal gas constant that has the same value for all gases—namely, R = 8.31 J/mol K.
The power of the ideal gas law is in its simplicity. When any two of the thermodynamic variables, p, v, and T are given, the third can easily be found. An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly elastic and in which there are no intermolecular attractive forces. An ideal gas can be visualized as a collection of perfectly hard spheres which collide but which otherwise do not interact with each other. In reality, no real gases are like an ideal gas and therefore no real gases follow the ideal gas law or equation completely. At temperatures near a gases boiling point, increases in pressure will cause condensation to take place and drastic decreases in volume. At very high pressures, the intermolecular forces of a gas are significant. However, most gases are in approximate agreement at pressures and temperatures above their boiling point. The ideal gas law is utilized by engineers working with gases because it is simple to use and approximates real gas behavior.
We hope, this article, Pressure Formula – Pressure Equation, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.