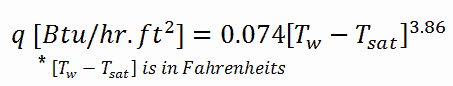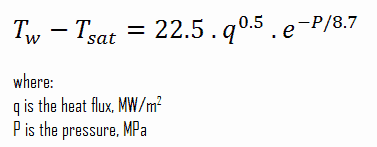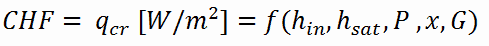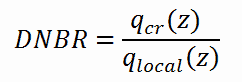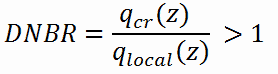Flow Boiling – Forced Convection Boiling
 In flow boiling (or forced convection boiling), fluid flow is forced over a surface by external means such as a pump, as well as by buoyancy effects. Therefore, flow boiling is always accompanied by other convection effects. Conditions depend strongly on geometry, which may involve external flow over heated plates and cylinders or internal (duct) flow. In nuclear reactors, most of boiling regimes are just forced convection boiling. The flow boiling is also classified as either external and internal flow boiling depending on whether the fluid is forced to flow over a heated surface or inside a heated channel.
In flow boiling (or forced convection boiling), fluid flow is forced over a surface by external means such as a pump, as well as by buoyancy effects. Therefore, flow boiling is always accompanied by other convection effects. Conditions depend strongly on geometry, which may involve external flow over heated plates and cylinders or internal (duct) flow. In nuclear reactors, most of boiling regimes are just forced convection boiling. The flow boiling is also classified as either external and internal flow boiling depending on whether the fluid is forced to flow over a heated surface or inside a heated channel.
Internal flow boiling is much more complicated in nature than external flow boiling because there is no free surface for the vapor to escape, and thus both the liquid and the vapor are forced to flow together. The two-phase flow in a tube exhibits different flow boiling regimes, depending on the relative amounts of the liquid and the vapor phases. Therefore internal forced convection boiling is commonly referred to as two-phase flow.
- In the case of steam and liquid water the density of the two phases differs by a factor of about 1000. Therefore the influence of gravitational body force on multiphase flows is of much greater importance than in the case of single-phase flows.
- The sound speed changes dramatically for materials undergoing phase change, and can be orders of magnitude different. This significantly influences a flow through an orifice.
- The relative concentration of different phases is usually a dependent parameter of great importance in multiphase flows, while it is a parameter of no consequence in single-phase flows.
- The change of phase means flow-induced pressure drops can cause further phase-change (e.g. water can evaporate through an orifice) increasing the relative volume of the gaseous, compressible medium and increasing efflux velocities, unlike single-phase incompressible flow where decreasing of an orifice would decrease efflux velocities.
- The spatial distribution of the various phases in the flow channel strongly affects the flow behavior.
- There are many types of instabilities in multiphase flow.
Flow Boiling – Vertical Channel
In this chapter, we will study flow boiling in a vertical channel of a boiling water reactor. The regimes of boiling and the heat flux curve are similar to the ones in pool boiling. The process occurs also in modern high pressure forced circulation boilers.
In BWRs there is a phenomenon, that is of the highest importance in reactor safety. This phenomenon is known as the “dryout” and it is directly associated with changes in flow pattern during evaporation. At normal the fuel surface is effectively cooled by boiling coolant. However when the heat flux exceeds a critical value (CHF – critical heat flux) the flow pattern may reach the dryout conditions (thin film of liquid disappears). The heat transfer from the fuel surface into the coolant is deteriorated, with the result of a drastically increased fuel surface temperature. In the high-quality region, the crisis occurs at a lower heat flux. Since the flow velocity in the vapor core is high, post-CHF heat transfer is much better than for low-quality critical flux (i.e. for PWRs temperature rises are higher and more rapid).
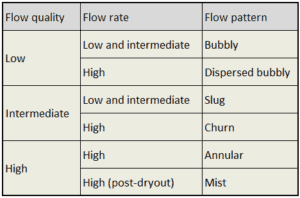
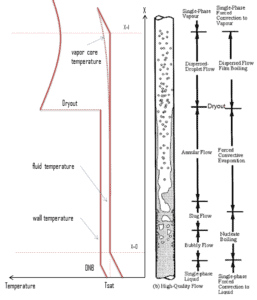 Typical flow boiling modes in a vertical channel are depicted in the figure. This figure shows the typical order of the flow regimes that are encountered from inlet to outlet of a heated channel. At the inlet, the liquid enters subcooled (at the lower temperature than saturation). In this region the flow is single-phase. As the liquid heats up, the wall temperature correspondingly rises. As the wall temperature exceeds the saturation temperature (e.g. 285°C at 6.8 MPa), subcooled nucleate boiling begins. Bubbles nucleate in the superheated thermal boundary layer on the heated wall but tend to condense in the subcooled bulk.
Typical flow boiling modes in a vertical channel are depicted in the figure. This figure shows the typical order of the flow regimes that are encountered from inlet to outlet of a heated channel. At the inlet, the liquid enters subcooled (at the lower temperature than saturation). In this region the flow is single-phase. As the liquid heats up, the wall temperature correspondingly rises. As the wall temperature exceeds the saturation temperature (e.g. 285°C at 6.8 MPa), subcooled nucleate boiling begins. Bubbles nucleate in the superheated thermal boundary layer on the heated wall but tend to condense in the subcooled bulk.
Further increase in liquid temperature causes, that the liquid bulk reaches its saturation temperature and the convective boiling process passes through the bubbly flow into the slug flow. Increasing void fraction causes that the structure of the flow becomes unstable. The boiling process passes through the slug and churn flow into the annular flow regime with its characteristic annular film of the liquid. At given combinations of flow rate through a channel, pressure, flow quality, and linear heat rate, the wall liquid film may exhaust and the wall may be dried out. At the dryout point the wall temperature significantly rises in order to dissipate the applied heat flux. The post-dryout flow (mist or drop flow) in the heated channel is undesirable, because the presence of such flow regime is accompanied with significantly higher wall temperatures and high fluctuation of wall temperatures. Correlations used to determine heat transfer coefficients in two phase flow are described below.
Special Reference: Tong, L. S., Weisman, Joel. Thermal Analysis of Pressurized Water Reactors. Amer Nuclear Society, 3rd edition, 5/1996. ISBN-13: 978-0894480386.
Single-phase Forced Convection – Heat Transfer Correlation
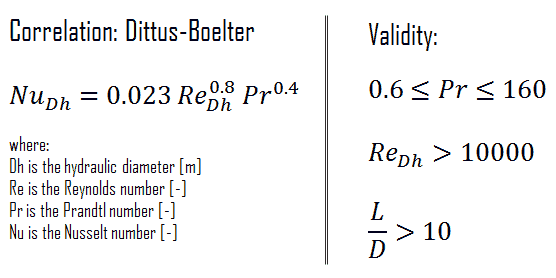
For fully developed (hydrodynamically and thermally) turbulent flow in a smooth circular tube, the local Nusselt number may be obtained from the well-known Dittus-Boelter equation. The DittusBoelter equation is easy to solve but is less accurate when there is a large temperature difference across the fluid and is less accurate for rough tubes (many commercial applications), since it is tailored to smooth tubes.
The Dittus-Boelter correlation may be used for small to moderate temperature differences, Twall – Tavg, with all properties evaluated at an averaged temperature Tavg.
For flows characterized by large property variations, the corrections (e.g. a viscosity correction factor μ/μwall) must be taken into account, for example, as Sieder and Tate recommend.
Nucleate Boiling Correlations – Flow Boiling
McAdams Correlation
In fully developed nucleate boiling with saturated coolant, the wall temperature is determined by local heat flux and pressure and is only slightly dependent on the Reynolds number. For subcooled water at absolute pressures between 0.1 – 0.6 MPa, McAdams correlation gives:
Thom Correlation
The Thom correlation is for the flow boiling (subcooled or saturated at pressures up to about 20 MPa) under conditions where the nucleate boiling contribution predominates over forced convection. This correlation is useful for rough estimation of expected temperature difference given the heat flux:
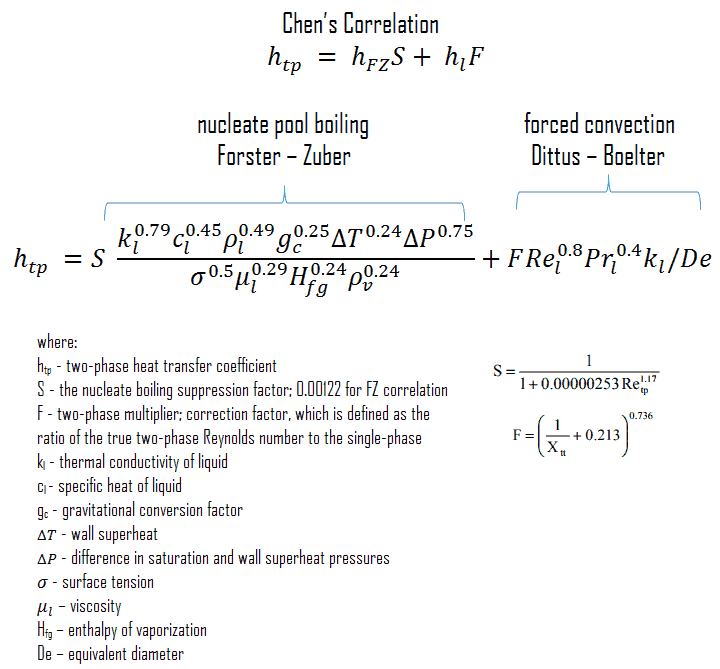
Chen’s Correlation
In 1963, Chen proposed the first flow boiling correlation for evaporation in vertical tubes to attain widespread use. Chen’s correlation includes both the heat transfer coefficients due to nucleate boiling as well as forced convective mechanisms. It must be noted, at higher vapor fractions, the heat transfer coefficient varies strongly with flow rate. The flow velocity in a core can be very high causing very high turbulences. This heat transfer mechanism has been referred to as “forced convection evaporation”. No adequate criteria has been established to determine the transition from nucleate boiling to forced convection vaporization. However, a single correlation that is valid for both nucleate boiling and forced convection vaporization has been developed by Chen for saturated boiling conditions and extended to include subcooled boiling by others. Chen proposed a correlation where the heat transfer coefficient is the sum of a forced convection component and a nucleate boiling component. It must be noted, the nucleate pool boiling correlation of Forster and Zuber (1955) is used to calculate the nucleate boiling heat transfer coefficient, hFZ and the turbulent flow correlation of Dittus-Boelter (1930) is used to calculate the liquid-phase convective heat transfer coefficient, hl.
The nucleate boiling suppression factor, S, is the ratio of the effective superheat to wall superheat. It accounts for decreased boiling heat transfer because the effective superheat across the boundary layer is less than the superheat based on wall temperature. The two-phase multiplier, F, is a function of the Martinelli parameter χtt.
Boiling Crisis – Critical Heat Flux
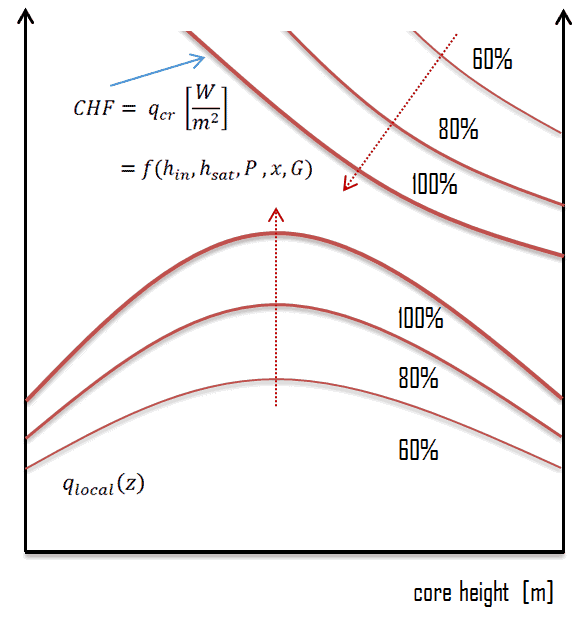
 As was written, in nuclear reactors, limitations of the local heat flux is of the highest importance for reactor safety. For pressurized water reactors and also for boiling water reactors, there are thermal-hydraulic phenomena, which cause a sudden decrease in the efficiency of heat transfer (more precisely in the heat transfer coefficient). These phenomena occur at certain value of heat flux, known as the “critical heat flux”. The phenomena, that cause the deterioration of heat transfer are different for PWRs and for BWRs.
As was written, in nuclear reactors, limitations of the local heat flux is of the highest importance for reactor safety. For pressurized water reactors and also for boiling water reactors, there are thermal-hydraulic phenomena, which cause a sudden decrease in the efficiency of heat transfer (more precisely in the heat transfer coefficient). These phenomena occur at certain value of heat flux, known as the “critical heat flux”. The phenomena, that cause the deterioration of heat transfer are different for PWRs and for BWRs.
In both types of reactors, the problem is more or less associated with departure from nucleate boiling. The nucleate boiling heat flux cannot be increased indefinitely. At some value, we call it the “critical heat flux” (CHF), the steam produced can form an insulating layer over the surface, which in turn deteriorates the heat transfer coefficient. Immediately after the critical heat flux has been reached, boiling become unstable and film boiling occurs. The transition from nucleate boiling to film boiling is known as the “boiling crisis”. As was written, the phenomena, that cause the deterioration of heat transfer are different for PWRs and for BWRs.
Departure From Nucleate Boiling – DNB
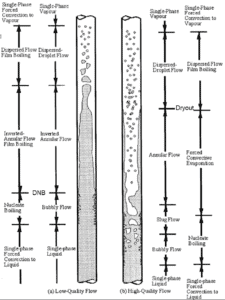
 In case of PWRs, the critical safety issue is named DNB (departure from nucleate boiling), which causes the formation of a local vapor layer, causing a dramatic reduction in heat transfer capability. This phenomenon occurs in the subcooled or low-quality region. The behaviour of the boiling crisis depends on many flow conditions (pressure, temperature, flow rate), but the boiling crisis occurs at a relatively high heat fluxes and appears to be associated with the cloud of bubbles, adjacent to the surface. These bubbles or film of vapor reduces the amount of incoming water. Since this phenomenon deteriorates the heat transfer coefficient and the heat flux remains, heat then accumulates in the fuel rod causing dramatic rise of cladding and fuel temperature. Simply, a very high temperature difference is required to transfer the critical heat flux being produced from the surface of the fuel rod to the reactor coolant (through vapor layer).
In case of PWRs, the critical safety issue is named DNB (departure from nucleate boiling), which causes the formation of a local vapor layer, causing a dramatic reduction in heat transfer capability. This phenomenon occurs in the subcooled or low-quality region. The behaviour of the boiling crisis depends on many flow conditions (pressure, temperature, flow rate), but the boiling crisis occurs at a relatively high heat fluxes and appears to be associated with the cloud of bubbles, adjacent to the surface. These bubbles or film of vapor reduces the amount of incoming water. Since this phenomenon deteriorates the heat transfer coefficient and the heat flux remains, heat then accumulates in the fuel rod causing dramatic rise of cladding and fuel temperature. Simply, a very high temperature difference is required to transfer the critical heat flux being produced from the surface of the fuel rod to the reactor coolant (through vapor layer).
In case of PWRs, the critical flow is inverted annular flow, while in BWRs, the critical flow is usually annular flow. The difference in flow regime between post-dryout flow and post-DNB flow is depicted in the figure. In PWRs at normal operation the flow is considered to be single-phase. But a great deal of study has been performed on the nature of two-phase flow in case of transients and accidents (such as the loss-of-coolant accident – LOCA or trip of RCPs), which are of importance in reactor safety and in must be proved and declared in the Safety Analysis Report (SAR).
In pressurized water reactors, one of key safety requirements is that a departure from nucleate boiling (DNB) will not occur during steady state operation, normal operational transients, and anticipated operational occurrences (AOOs). Fuel cladding integrity will be maintained if the minimum DNBR remains above the 95/95 DNBR limit for PWRs ( a 95% probability at a 95% confidence level). DNB criterion is one of acceptance criteria in safety analyses as well as it constitutes one of safety limits in technical specifications.
Critical Heat Flux for DNB – Correlations
As was written, the boiling crisis can be classified as dryout (will be described below DNB) in the high-quality region and departure from nucleate boiling (DNB) in the subcooled or low-quality region (approximate quality range: from –5% to +5%). But the critical heat flux is used for both regimes.
DNB – W-3 Correlation
One of the most well known design correlations for predicting departure from nucleate boiling is the W-3 correlation developed at the Westinghouse Atomic Power Division by Tong. It is applicable for subcooled and low to moderate quality flows.The W-3 correlation is a function of coolant enthalpy (saturated and inlet), pressure, quality and coolant mass flux:
The correlation W-3 is for critical heat flux in uniformly heated channels. To account for non-uniform heat fluxes, Tong introduced the correction factor, F.
Special Reference: Tong, L. S., Weisman, Joel. Thermal Analysis of Pressurized Water Reactors. Amer Nuclear Society, 3rd edition, 5/1996. ISBN-13: 978-0894480386.
Cold Wall Factor – CWF
Tong, L. S. and Weisman, Joel also introduces a new factor known as the “cold wall factor”, which corrects CHF in a channel containing an unheated wall (e.g. channel adjacent to control rod guide tube). In these channels, liquid film builds up along the cold wall and this fluid is not effective in cooling the heated surface and the fluid cooling the heated surface is at higher enthalpy than calculated without assumption of cold wall. Note that, there is an assumption that cold wall deteriorates heat transfer compared to channel with all sides heated at the same bulk exit enthalpy.
CHF Look-up Tables
CHF look-up tables are used widely for the prediction of the critical heat flux (CHF). The CHF look-up table is basically a normalized data bank for a vertical 8 mm water-cooled tube. The 2006 CHF look-up table is based on a database containing more than 30,000 data points and they cover the ranges of 0.1–21 Mpa pressure, 0–8000 kg.m–2.s-1 (zero flow refers to pool-boiling conditions) mass flux and –0.5 to 1 vapour quality (negative qualities refer to subcooled conditions).
Special Reference: GROENEVELD, D.C. et al., The 2006 look-up table, Nuclear Engineering and Design 237 (2007), 1909–1922.
Departure from Nucleate Boiling Ratio – DNBR
As was written, in case of PWRs, the critical safety issue is named DNB (departure from nucleate boiling), which causes the formation of a local vapor layer, causing a dramatic reduction in heat transfer capability. Note that, even for BWRs, which have a significantly bottom-peaked axial power profile, the DNB-risk have to be taken into account.
DNB occurs, when the local heat flux reaches value of critical heat flux. This phenomenon occurs in the subcooled or low-quality region (approximate quality range: from –5% to +5%). The behaviour of this type of boiling crisis depends on many flow conditions (pressure, temperature, flow rate), since the critical heat flux is generally a function of coolant enthalpy (saturated and inlet), pressure, quality and coolant mass flux:
This type of boiling crisis occurs at a relatively high heat fluxes and appears to be associated with the cloud of bubbles, adjacent to the surface. These bubbles or film of vapor reduces the amount of incoming water. Since this phenomenon deteriorates the heat transfer coefficient and the heat flux remains, heat then accumulates in the fuel rod causing dramatic rise of cladding and fuel temperature. Simply, a very high temperature difference is required to transfer the critical heat flux being produced from the surface of the fuel rod to the reactor coolant (through vapor layer). In case of PWRs, the critical flow is inverted annular flow, while in BWRs, the critical flow is usually annular flow.
In pressurized water reactors, one of key safety requirements is that a departure from nucleate boiling (DNB) will not occur during steady state operation, normal operational transients, and anticipated operational occurrences (AOOs). Fuel cladding integrity will be maintained if the minimum DNBR remains above the 95/95 DNBR limit for PWRs ( a 95% probability at a 95% confidence level). DNB criterion is one of acceptance criteria in safety analyses as well as it constitutes one of safety limits in technical specifications. Needless to say, the establishment of a minimum DNB ratio provides a major limitation on the design of water cooled reactors. This phenomenon limits the maximal thermal power of each PWR.
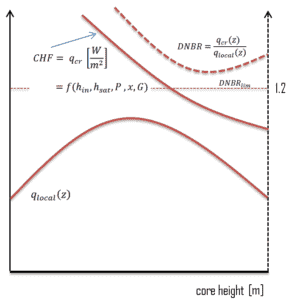
DNB ratio (DNBR – Departure from Nucleate Boiling Ratio) is the measure of the margin to critical heat flux. DNBR is defined as:
the critical heat flux at a specific location and specific coolant parameters divided by the operating local heat flux at that location.
The reactor core must be designed to keep the DNBR larger than the minimum allowable value (known as the correlation limit) during steady state operation, normal operational transients, and anticipated operational occurrences (AOOs). For predicting departure from nucleate boiling, CHF can be, for example, determined using the W-3 correlation developed at the Westinghouse Atomic Power Division. If these correlation were perfect (without uncertainties), the criterion would be simple:
Local heat flux must be lower than critical heat flux (i.e. DNBR must be higher than one).
 But in reality, no correlation is perfect and uncertainties must be involved in this calculation. These uncertainty bands or error bounds establish a minimum acceptable value for the DNB Ratio, which may be significantly greater than one as indicated in the figure. Uncertainties may reach about 20% and therefore the DNBR must be larger than, for example, DNBRlim = 1,2.
But in reality, no correlation is perfect and uncertainties must be involved in this calculation. These uncertainty bands or error bounds establish a minimum acceptable value for the DNB Ratio, which may be significantly greater than one as indicated in the figure. Uncertainties may reach about 20% and therefore the DNBR must be larger than, for example, DNBRlim = 1,2.
As can be seen from the figure, the CHF significantly decreases with increasing coolant enthalpy, therefore minimal value of DNBR is not necessarily in the center of the core. The Minimum DNB Ratio (MDNBR) occurs at the location where the critical heat flux and the operating heat flux are the closest and it is usually in the upper part of the core. Moreover, at the channel inlet where the coolant subcooling is the highest, we would expect the heat flux necessary to cause DNB at this location to be extremely high. On the other hand, at the channel exit where the coolant enthalpy is its highest, the heat flux necessary to cause DNB should be at its lowest.
Special Reference: Tong, L. S., Weisman, Joel. Thermal Analysis of Pressurized Water Reactors. Amer Nuclear Society, 3rd edition, 5/1996. ISBN-13: 978-0894480386.
Post-DNB Heat Transfer
The nucleate boiling heat flux cannot be increased indefinitely. At some value, we call it the “critical heat flux” (CHF), the steam produced can form an insulating layer over the surface, which in turn deteriorates the heat transfer coefficient. This is because a large fraction of the surface is covered by a vapor film, which acts as an thermal insulation due to the low thermal conductivity of the vapor relative to that of the liquid. Immediately after the critical heat flux has been reached, boiling become unstable and transition boiling occurs. The transition from nucleate boiling to film boiling is known as the “boiling crisis”. Since beyond the CHF point the heat transfer coefficient decreases, the transition to film boiling is usually inevitable.
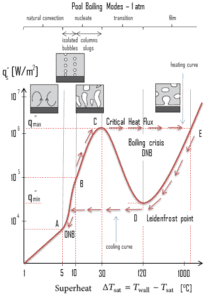 A further increase in the heat flux is not necessary to maintain film boiling. A film of vapour fully covers the surface. This significantly reduces the convection coefficient, since the vapor layer has significantly lower heat transfer ability. As a result the excess temperature shoots up to a very high value. Beyond the Leidenfrost point, a continuous vapor film blankets the surface and there is no contact between the liquid phase and the surface. In this situation the heat transfer is both by radiation and by conduction to the vapour. Heated surface stabilizes stabilizes its temperature at point E (see figure). If the material is not strong enough for withstanding this temperature, the equipment will fail by damage to the material.
A further increase in the heat flux is not necessary to maintain film boiling. A film of vapour fully covers the surface. This significantly reduces the convection coefficient, since the vapor layer has significantly lower heat transfer ability. As a result the excess temperature shoots up to a very high value. Beyond the Leidenfrost point, a continuous vapor film blankets the surface and there is no contact between the liquid phase and the surface. In this situation the heat transfer is both by radiation and by conduction to the vapour. Heated surface stabilizes stabilizes its temperature at point E (see figure). If the material is not strong enough for withstanding this temperature, the equipment will fail by damage to the material.
Critical Power Ratio – Dryout
 In BWRs, similar phenomenon is known as “dryout” and it is directly associated with changes in flow pattern during evaporation in the high-quality region. At given combinations of flow rate through a channel, pressure, flow quality, and linear heat rate, the wall liquid film may exhaust and the wall may be dried out. At normal, the fuel surface is effectively cooled by boiling coolant. However when the heat flux exceeds a critical value (CHF – critical heat flux) the flow pattern may reach the dryout conditions (thin film of liquid disappears). The heat transfer from the fuel surface into the coolant is deteriorated, with the result of a drastically increased fuel surface temperature. In the high-quality region, the crisis occurs at a lower heat flux. Since the flow velocity in the vapor core is high, post-CHF heat transfer is much better than for low-quality critical flux (i.e. for PWRs temperature rises are higher and more rapid).
In BWRs, similar phenomenon is known as “dryout” and it is directly associated with changes in flow pattern during evaporation in the high-quality region. At given combinations of flow rate through a channel, pressure, flow quality, and linear heat rate, the wall liquid film may exhaust and the wall may be dried out. At normal, the fuel surface is effectively cooled by boiling coolant. However when the heat flux exceeds a critical value (CHF – critical heat flux) the flow pattern may reach the dryout conditions (thin film of liquid disappears). The heat transfer from the fuel surface into the coolant is deteriorated, with the result of a drastically increased fuel surface temperature. In the high-quality region, the crisis occurs at a lower heat flux. Since the flow velocity in the vapor core is high, post-CHF heat transfer is much better than for low-quality critical flux (i.e. for PWRs temperature rises are higher and more rapid).
In this case, engineers define parameter known as the minimum critical power ratio (MCPR) instead of DNBR. The critical power ratio (CPR) is used for determining the thermal limits of boiling water reactors.
Definition of CPR :
The CPR is that power in the assembly that is calculated by application of the appropriate correlation(s) to cause some point in the assembly to experience boiling transition, divided by the actual assembly operating power.
We hope, this article, Flow Boiling – Forced Convection Boiling, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.
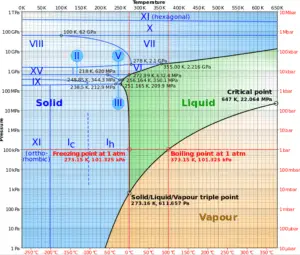
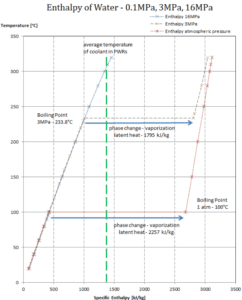
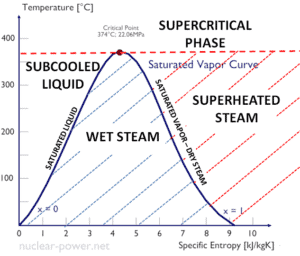 The change from the liquid to the vapor state due to boiling is sustained by heat transfer from the solid surface; conversely, condensation of a vapor to the liquid state results in heat transfer to the solid surface. Boiling and condensation differ from other forms of convection in that they depend on the latent heat of vaporization, which is very high for common
The change from the liquid to the vapor state due to boiling is sustained by heat transfer from the solid surface; conversely, condensation of a vapor to the liquid state results in heat transfer to the solid surface. Boiling and condensation differ from other forms of convection in that they depend on the latent heat of vaporization, which is very high for common