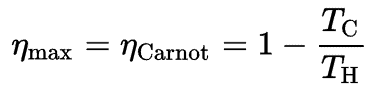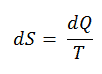Various Statements of the Second Law of Thermodynamics
The second law of thermodynamics may be expressed in many specific ways. Each statement expresses the same law. Listed below are three that are often encountered.
Before these statements, we have to remind the work of a French engineer and physicist, Nicolas Léonard Sadi Carnot advanced the study of the second law by forming a principle (also called Carnot’s rule) that specifies limits on the maximum efficiency any heat engine can obtain.
Clausius Statement of the Second Law
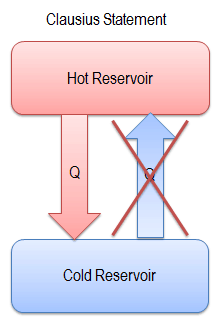
“It is impossible to construct a device which operates on a cycle and whose sole effect is the transfer of heat from a cooler body to a hotter body”.
Heat cannot spontaneously flow from cold system to hot system without external work being performed on the system. This is exactly what refrigerators and heat pumps accomplish. In a refrigerator, heat flows from cold to hot, but only when forced by an external work, refrigerators are driven by electric motors requiring work from their surroundings to operate.
The Clausius and the Kelvin-Planck statements have been shown to be equivalent.
Kelvin-Planck Statement of the Second Law
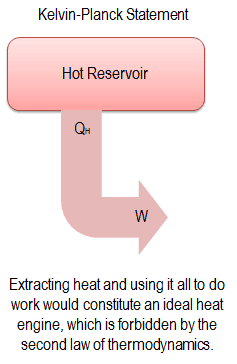 “It is impossible to construct a device which operates on a cycle and produces no other effect than the production of work and the transfer of heat from a single body”.
“It is impossible to construct a device which operates on a cycle and produces no other effect than the production of work and the transfer of heat from a single body”.
This statement operates with the term “thermal reservoir” or “single reservoir”. A reservoir is a large object, in which the temperature remains constant while energy is extracted. Such a system can be approximated in a number of ways—by the earth’s atmosphere, large bodies of water like lakes, oceans and so on.
The Kelvin–Planck statement does not exclude the existence of a system, that develops a net amount of work from a heat transfer extracted from a thermal reservoir. According to this statement, a system undergoing a cycle cannot develop a positive net amount of work from a heat transfer extracted from a thermal reservoir.
Entropy and the Second Law
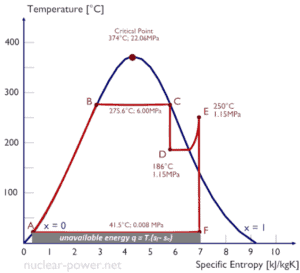
One consequence of the second law of thermodynamics is the development of the physical property of matter, that is known as the entropy (S). The change in this property is used to determine the direction in which a given process will proceed. Entropy quantifies the energy of a substance that is no longer available to perform useful work. This relates to the second law since the second law predicts that not all heat provided to a cycle can be transformed into an equal amount of work, some heat rejection must take place.
See also: Entropy
According to Clausius, the entropy was defined via the change in entropy S of a system. The change in entropy S, when an amount of heat Q is added to it by a reversible process at constant temperature, is given by:
Here Q is the energy transferred as heat to or from the system during the process, and T is the temperature of the system in kelvins during the process. The SI unit for entropy is J/K.
The second law of thermodynamics can also be expressed as ∆S≥0 for a closed cycle.
In words:
The entropy of any isolated system never decreases. In a natural thermodynamic process, the sum of the entropies of the interacting thermodynamic systems increases.
∆S≥0
Because entropy tells so much about the usefulness of an amount of heat transferred in performing work, the steam tables include values of specific entropy (s = S/m) as part of the information tabulated.
We hope, this article, Statement of the Second Law, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.