What is Temperature
In physics and in everyday life a temperature is an objective comparative measurement of hot or cold based on our sense of touch. A body that feels hot usually has a higher temperature than a similar body that feels cold. But this definition is not a simple matter. For example, a metal rod feels colder than a plastic rod at room temperature simply because metals are generally better at conducting heat away from the skin as are plastics. Simply hotness may be represented abstractly and therefore it is necessary to have an objective way of measuring temperature. It is one of basic thermodynamic properties. What is the temperature in physics….
Thermal Equilibrium
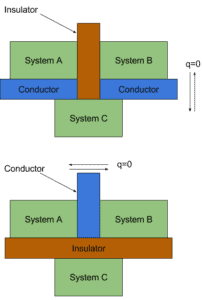
A particularly important concept is thermodynamic equilibrium. In general, when two objects are brought into thermal contact, heat will flow between them until they come into equilibrium with each other. When a temperature difference does exist heat flows spontaneously from the warmer system to the colder system. Heat transfer occurs by conduction or by thermal radiation. When the flow of heat stops, they are said to be at the same temperature. They are then said to be in thermal equilibrium.
For example, you leave a thermometer in a cup of coffee. As the two objects interact, the thermometer becomes hotter and the coffee cools off a little until they come into thermal equilibrium. Two objects are defined to be in thermal equilibrium if, when placed in thermal contact, no net energy flows from one to the other, and their temperatures don’t change. We may postulate:
When the two objects are in thermal equilibrium, their temperatures are equal.
This is a subject of a law that is called the “zeroth law of thermodynamics”.
For example, most materials expand when their temperature is increased. This property is very important in all of the science and engineering, even in nuclear engineering. The thermodynamic efficiency of power plants changes with temperature of inlet steam or even with outside temperature. At higher temperatures, solids such as steel glow orange or even white depending on temperature. The white light from an ordinary incandescent lightbulb comes from an extremely hot tungsten wire. It can be seen temperature is one of the fundamental characteristics that describes matter and influences matter behaviour.
Temperature Scales
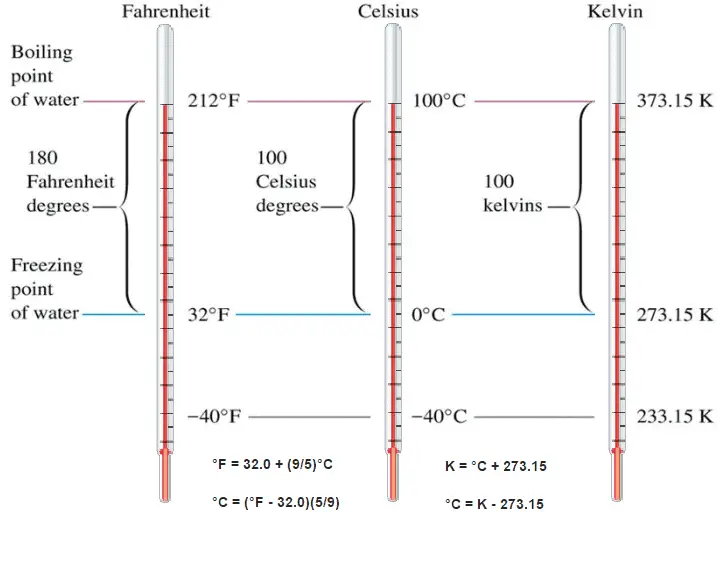
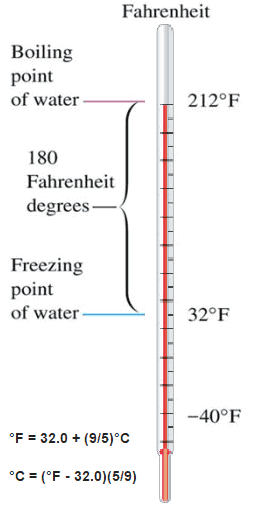
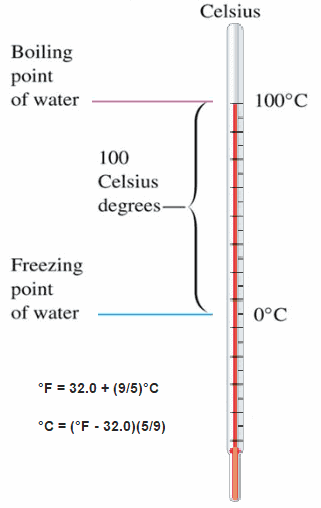
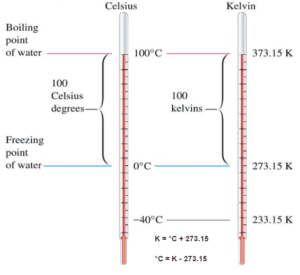
These numbers are arbitrary, and historically many different schemes have been used. For example, this was done by defining some physical occurrences at given temperatures—such as the freezing and boiling points of water — and defining them as 0 and 100 respectively.
There are several scales and units exist for measuring temperature. The most common are:
- Celsius (denoted °C),
- Fahrenheit (denoted °F),
- Kelvin (denoted K; especially in science).
Temperature required nuclear fusion to occur
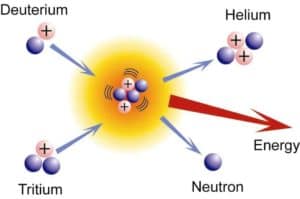 Deuterium-Tritium Fusion. The fusion reaction of deuterium and tritium is particularly interesting because of its potential of providing energy for the future.
Deuterium-Tritium Fusion. The fusion reaction of deuterium and tritium is particularly interesting because of its potential of providing energy for the future.
3T (d, n) 4He
The reaction yields ~17 MeV of energy per reaction but requires a enormous temperature of approximately 40 million Kelvins to overcome the coulomb barrier by the attractive nuclear force, which is stronger at close distances. The deuterium fuel is abundant, but tritium must be either bred from lithium or gotten in the operation of the deuterium cycle.
Highest man-made temperature
On 13 August 2012 scientists at CERN’s Large Hadron Collider (LHC), Geneva, Switzerland, announced that they created a quark-gluon plasma with a record-smashing temperature of 5.5 trillion K.
The team had been using the ALICE experiment, that is focused on studying the QCP and other conditions in the primordial universe, to smash together lead ions at 99% of the speed of light to create a quark gluon plasma. It is believed that up to a few milliseconds after the Big Bang, the Universe was in a quark–gluon plasma state, which is an exotic state of matter. It is thought quark–gluon plasma consist of asymptotically free quarks and gluons.
Lowest man-made temperature
According to Guiness World Records the lowest manmade temperature achieved to date is 450 picokelvin above absolute zero (only half-a-billionth of a degree above absolute zero). It was achieved by a team of scientists at the Massachusetts Institute of Technology, Cambridge, Massachusetts, USA.
We hope, this article, Temperature – Physics, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.