Conduction – Convection – Radiation
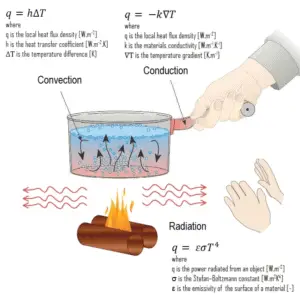 Heat transfer is an engineering discipline that concerns the generation, use, conversion, and exchange of heat (thermal energy) between physical systems. In power engineering it determines key parameters and materials of heat exchangers. Heat transfer is usually classified into various mechanisms, such as:
Heat transfer is an engineering discipline that concerns the generation, use, conversion, and exchange of heat (thermal energy) between physical systems. In power engineering it determines key parameters and materials of heat exchangers. Heat transfer is usually classified into various mechanisms, such as:
- Heat Conduction. Heat conduction, also called diffusion, occurs within a body or between two bodies in contact. It is the direct microscopic exchange of kinetic energy of particles through the boundary between two systems. When an object is at a different temperature from another body or its surroundings
- Heat Convection. Heat convection depends on motion of mass from one region of space to another. Heat convection occurs when bulk flow of a fluid (gas or liquid) carries heat along with the flow of matter in the fluid.
- Thermal Radiation. Radiation is heat transfer by electromagnetic radiation, such as sunshine, with no need for matter to be present in the space between bodies.
Conduction
Thermal conduction, also called heat conduction, occurs within a body or between two bodies in contactwithout the involvement of mass flow and mixing. It is the direct microscopic exchange of kinetic energy of particles through the boundary between two systems. Heat transfer by conductionis dependent upon the driving “force” of temperature difference and the thermal conductivity (or the resistance to heat transfer). The thermal conductivity is dependent upon the nature and dimensions of the heat transfer medium. All heat transfer problems involve the temperature difference, the geometry, and the physical propertiesof the object being studied. In conduction heat transfer problems, the object being studied is usually a solid.
Microscopically this mode of energy transfer is attributed to free electron flow from higher to lower energy levels, lattice vibration and molecular collision. Consider a block of stone at high temperature, that consists of atoms that are oscillating intensely around their average positions. At low temperatures, the atoms continue to oscillate, but with less intensity. If a hotter block of stone is put in contact with a cooler block, the intensely oscillating atoms at the edge of the hotter block gives off its kinetic energy to the less oscillating atoms at the edge of the cool block. In this case there is energy transfer between these two blocks and heat flows from the hotter to the cooler block by this random vibrations. The modern view is to ascribe the energy transfer to lattice waves induced by atomic motion. In an electrical insulators, the energy transfer is exclusively via these lattice waves. In a conductor, it is also due to the translational motion of the free electrons.
Convection
In general, convection is either the mass transfer or the heat transfer due to bulk movement of molecules within fluids such as gases and liquids. Although liquids and gases are generally not very good conductors of heat, they can transfer heat quite rapidly by convection.
Convection takes place through advection, diffusion or both. Convection cannot take place in most solids because neither significant diffusion of matter nor bulk current flows can take place. Diffusion of heat takes place in rigid solids, but that is called thermal conduction.
The process of heat transfer between a surface and a fluid flowing in contact with it is called convective heat transfer. In engineering, convective heat transfer is one of the major mechanisms of heat transfer. When heat is to be transferred from one fluid to another through a barrier, convection is involved on both sides of the barrier. In most cases the main resistance to heat flow is by convection. Convective heat transfer take place both by thermal diffusion (the random motion of fluid molecules) and by advection, in which matter or heat is transported by the larger-scale motion of currents in the fluid.
Radiation Heat Transfer
In preceding chapters, we have discussed convection and conduction, which require the presence of matter as a medium to carry the heat from the hotter to the colder region. But a third type of heat transfer, radiation heat transfer, occurs without any medium at all. In general, the radiation heat transfer from one surface to another is the radiation leaving the first surface for the other minus that arriving from the second surface. Radiation heat transfer is mediated by electromagnetic radiation, known as thermal radiation, that arises due to the temperature of a body. Any material that has a temperature above absolute zero gives off some radiant energy. Most energy of this type is in the infra-red region of the electromagnetic spectrum although some of it is in the visible region. One of most important examples of radiation heat transfer is the Earth’s absorption of solar radiation, followed by its outgoing thermal radiation. These processes determine the temperature and climate of the Earth.
Source: hyperphysics.phy-astr.gsu.edu
We hope, this article, Conduction – Convection – Radiation, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.