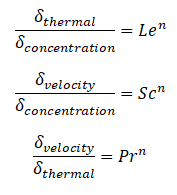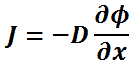What is Lewis Number
The Lewis number is a dimensionless number, named after Warren K. Lewis (1882–1975). The Lewis number is defined as the ratio of thermal diffusivity and mass diffusivity. It is used to characterize fluid flows where there is simultaneous heat and mass transfer. The Lewis number is therefore a measure of the relative thermal and concentration boundary layer thicknesses. The Lewis number can also be expressed in terms of the Prandtl number and the Schmidt number as Le = Sc / Pr.
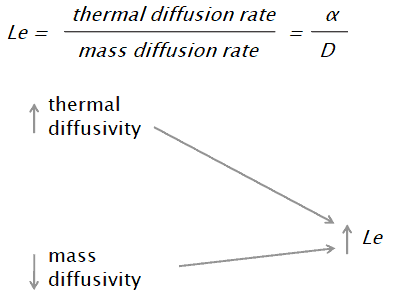
The Lewis number is defined as:
where:
α is thermal diffusivity [m2/s]
D is the mass diffusivity [m2/s]
Similarly as for Schmidt and Prandtl Number, the Lewis number physically relates the relative thickness of the thermal layer and mass-transfer (concentration) boundary layer.
where n = 1/3 for most applications in all three relations. These relations, in general, are applicable only for laminar flow and are not applicable to turbulent boundary layers since turbulent mixing in this case may dominate the diffusion processes.
A Lewis number of unity indicates that thermal boundary layer and mass transfer by diffusion are comparable, and temperature and concentration boundary layers almost coincide with each other. Mass diffusivity or diffusion coefficient is a proportionality constant between the molar flux due to molecular diffusion and the gradient in the concentration of the species (or the driving force for diffusion).
Diffusivity is encountered in Fick’s law, which states:
If the concentration of a solute in one region is greater than in another of a solution, the solute diffuses from the region of higher concentration to the region of lower concentration, with a magnitude that is proportional to the concentration gradient.
In one (spatial) dimension, the law is:
where:
- J is the diffusion flux,
- D is the diffusion coefficient,
- φ (for ideal mixtures) is the concentration.
The use of this law in nuclear reactor theory leads to the diffusion approximation.
We hope, this article, Lewis Number, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.