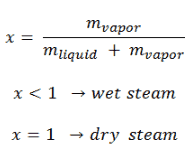Boiling Water
 Water is a transparent and nearly colorless substance composed of the chemical elements hydrogen and oxygen, that are connected by covalent bonds. On Earth, water exists in gaseous (steam or water vapor), liquid, and solid states (ice). It is one of the most plentiful and essential of compounds and it is the main constituent of Earth’s streams, lakes, and oceans, and the fluids of most living organisms. It is vital for all known forms of life.
Water is a transparent and nearly colorless substance composed of the chemical elements hydrogen and oxygen, that are connected by covalent bonds. On Earth, water exists in gaseous (steam or water vapor), liquid, and solid states (ice). It is one of the most plentiful and essential of compounds and it is the main constituent of Earth’s streams, lakes, and oceans, and the fluids of most living organisms. It is vital for all known forms of life.
Besides being essential to life, water is a remarkable substance with many surprising properties.
- It is the only chemical compound that occurs naturally in all three physical states – gaseous (steam or water vapor), liquid, and solid states (ice).
- It is the only inorganic liquid that occurs naturally on the Earth.
- Water also differs from most liquids in that it becomes less dense as it freezes. It has a maximum of density at 3.98 °C (1000 kg/m3), whereas the density of ice is 917 kg/m3. It differs by about 9% and therefore ice floats on liquid water
- Water has the highest specific heat of any common substance – 4.19 kJ/kg K.
- It has very high heat of vaporization, which makes it an effective coolant and medium in thermal power plants and other energy industry.
Source: wikipedia.org CC BY-SA
In preceding chapters, we have discussed convective heat transfer with very important assumption. We have assumed a single-phase convective heat transfer without any phase change. In this chapter we focus on convective heat transfer associated with the change in phase of a fluid. In particular, we consider processes that can occur at a solid–liquid or solid–vapor interface, namely, boiling (liquid-to-vapor phase change) and condensation (vapor-to-liquid phase change).
For these cases latent heat effects associated with the phase change are significant. Latent heat, known also as the enthalpy of vaporization, is the amount of heat added to or removed from a substance to produce a change in phase. This energy breaks down the intermolecular attractive forces, and also must provide the energy necessary to expand the gas (the pΔV work). When latent heat is added, no temperature change occurs.
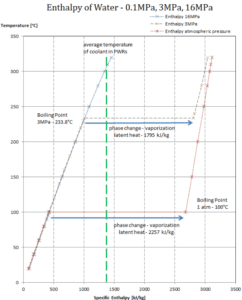
The enthalpy of vaporization is a function of the pressure at which that transformation takes place.
Latent heat of vaporization – water at 0.1 MPa (atmospheric pressure)
hlg = 2257 kJ/kg
Latent heat of vaporization – water at 3 MPa
hlg = 1795 kJ/kg
Latent heat of vaporization – water at 16 MPa (pressure inside a pressurizer)
hlg = 931 kJ/kg
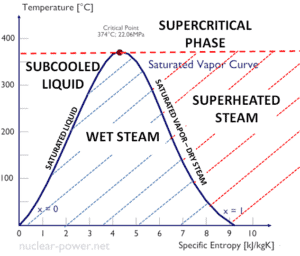
The heat of vaporization diminishes with increasing pressure, while the boiling point increases. It vanishes completely at a certain point called the critical point. Above the critical point, the liquid and vapor phases are indistinguishable, and the substance is called a supercritical fluid.
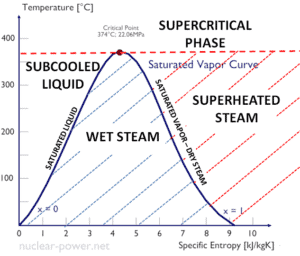 The change from the liquid to the vapor state due to boiling is sustained by heat transfer from the solid surface; conversely, condensation of a vapor to the liquid state results in heat transfer to the solid surface. Boiling and condensation differ from other forms of convection in that they depend on the latent heat of vaporization, which is very high for common pressures, therefore large amounts of heat can be transferred during boiling and condensation essentially at constant temperature. Heat transfer coefficients, h, associated with boiling and condensation are typically much higher than those encountered in other forms of convection processes that involve a single phase.
The change from the liquid to the vapor state due to boiling is sustained by heat transfer from the solid surface; conversely, condensation of a vapor to the liquid state results in heat transfer to the solid surface. Boiling and condensation differ from other forms of convection in that they depend on the latent heat of vaporization, which is very high for common pressures, therefore large amounts of heat can be transferred during boiling and condensation essentially at constant temperature. Heat transfer coefficients, h, associated with boiling and condensation are typically much higher than those encountered in other forms of convection processes that involve a single phase.
This is due to the fact, even in turbulent flow, there is a stagnant fluid film layer (laminar sublayer), that isolates the surface of the heat exchanger. This stagnant fluid film layer plays crucial role for the convective heat transfer coefficient. It is observed, that the fluid comes to a complete stop at the surface and assumes a zero velocity relative to the surface. This phenomenon is known as the no-slip condition and therefore, at the surface, energy flow occurs purely by conduction. But in the next layers both conduction and diffusion-mass movement in the molecular level or macroscopic level occurs. Due to the mass movement the rate of energy transfer is higher. As was written, nucleate boiling at the surface effectively disrupts this stagnant layer and therefore nucleate boiling significantly increases the ability of a surface to transfer thermal energy to bulk fluid.
Boiling Modes – Types of Boiling
 From a practical engineering point of view boiling can be categorized according to several criteria.
From a practical engineering point of view boiling can be categorized according to several criteria.
Categorization by the flow regime:
- Pool Boiling. Perhaps the most common configuration, known as pool boiling is when a pool of liquid is heated from below through a horizontal surface. In pool boiling the liquid is quiescent and its motion near the surface is primarily due to natural convection and to mixing induced by bubble growth and detachment. The pioneering work on pool boiling was done in 1934 by S. Nukiyama. He was the first to identify four well known different regimes of pool boiling using his apparatus.
 Flow Boiling. In flow boiling (or forced convection boiling), fluid flow is forced over a surface by external means such as a pump, as well as by buoyancy effects. Therefore, flow boiling is always accompanied by other convection effects. Conditions depend strongly on geometry, which may involve external flow over heated plates and cylinders or internal (duct) flow. In nuclear reactors, most of boiling regimes are just forced convection boiling.
Flow Boiling. In flow boiling (or forced convection boiling), fluid flow is forced over a surface by external means such as a pump, as well as by buoyancy effects. Therefore, flow boiling is always accompanied by other convection effects. Conditions depend strongly on geometry, which may involve external flow over heated plates and cylinders or internal (duct) flow. In nuclear reactors, most of boiling regimes are just forced convection boiling.
Categorization by the wall superheat temperature, ΔTsat:
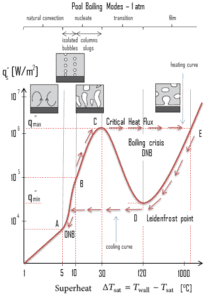 The pioneering work on boiling was done in 1934 by S. Nukiyama, who used electrically heated nichrome and platinum wires immersed in liquids in his experiments. Nukiyama was the first to identify different regimes of pool boiling using his apparatus. He noticed that boiling takes different forms, depending on the value of the wall superheat temperature ΔTsat (known also as the excess temperature), which is defined as the difference between the wall temperature, Twall and the saturation temperature, Tsat.
The pioneering work on boiling was done in 1934 by S. Nukiyama, who used electrically heated nichrome and platinum wires immersed in liquids in his experiments. Nukiyama was the first to identify different regimes of pool boiling using his apparatus. He noticed that boiling takes different forms, depending on the value of the wall superheat temperature ΔTsat (known also as the excess temperature), which is defined as the difference between the wall temperature, Twall and the saturation temperature, Tsat.
Four different boiling regimes of pool boiling (based on the excess temperature) are observed:
- Natural Convection Boiling ΔTsat < 5°C
- Nucleate Boiling 5°C < ΔTsat < 30°C
- Transition Boiling 30°C < ΔTsat < 200°C
- Film Boiling 200°C < ΔTsat
Description of Boiling Modes:
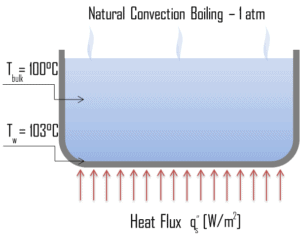 Natural Convection Boiling. In thermodynamics, the requirement for boiling of pure substances to occur is that Twall = Tsat. But in real experiments, boiling does not occur until the liquid is heated a few degrees above the saturation temperature. The surface temperature must be somewhat above the saturation temperature in order to sustain vapor formation. In this boiling mode, vapour will be observed over the water surface, but usually no bubbles will be observed. As the superheat temperature is increased, bubble inception will eventually occur, but below point A, fluid motion is determined principally by natural convection currents. The point A is usually referred to as the onset of nucleate boiling – ONB.
Natural Convection Boiling. In thermodynamics, the requirement for boiling of pure substances to occur is that Twall = Tsat. But in real experiments, boiling does not occur until the liquid is heated a few degrees above the saturation temperature. The surface temperature must be somewhat above the saturation temperature in order to sustain vapor formation. In this boiling mode, vapour will be observed over the water surface, but usually no bubbles will be observed. As the superheat temperature is increased, bubble inception will eventually occur, but below point A, fluid motion is determined principally by natural convection currents. The point A is usually referred to as the onset of nucleate boiling – ONB.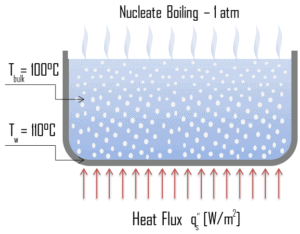 Nucleate Boiling. The most common type of local boiling encountered in nuclear facilities is nucleate boiling. In nucleate boiling, steam bubbles form at the heat transfer surface and then break away and are carried into the main stream of the fluid. Such movement enhances heat transfer because the heat generated at the surface is carried directly into the fluid stream. Once in the main fluid stream, the bubbles collapse because the bulk temperature of the fluid is not as high as the heat transfer surface temperature where the bubbles were created. This heat transfer process is sometimes desirable because the energy created at the heat transfer surface is quickly and efficiently “carried” away.
Nucleate Boiling. The most common type of local boiling encountered in nuclear facilities is nucleate boiling. In nucleate boiling, steam bubbles form at the heat transfer surface and then break away and are carried into the main stream of the fluid. Such movement enhances heat transfer because the heat generated at the surface is carried directly into the fluid stream. Once in the main fluid stream, the bubbles collapse because the bulk temperature of the fluid is not as high as the heat transfer surface temperature where the bubbles were created. This heat transfer process is sometimes desirable because the energy created at the heat transfer surface is quickly and efficiently “carried” away.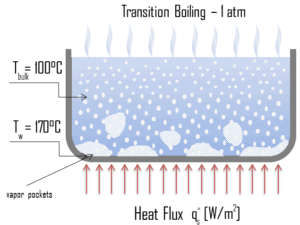 Transition Boiling. The nucleate boiling heat flux cannot be increased indefinitely. At some value, we call it the “critical heat flux” (CHF), the steam produced can form an insulating layer over the surface, which in turn deteriorates the heat transfer coefficient. This is because a large fraction of the surface is covered by a vapor film, which acts as an thermal insulation due to the low thermal conductivity of the vapor relative to that of the liquid. Immediately after the critical heat flux has been reached, boiling become unstable and transition boiling occurs. The transition from nucleate boiling to film boiling is known as the “boiling crisis”. Since beyond the CHF point the heat transfer coefficient decreases, the transition to film boiling is usually inevitable.
Transition Boiling. The nucleate boiling heat flux cannot be increased indefinitely. At some value, we call it the “critical heat flux” (CHF), the steam produced can form an insulating layer over the surface, which in turn deteriorates the heat transfer coefficient. This is because a large fraction of the surface is covered by a vapor film, which acts as an thermal insulation due to the low thermal conductivity of the vapor relative to that of the liquid. Immediately after the critical heat flux has been reached, boiling become unstable and transition boiling occurs. The transition from nucleate boiling to film boiling is known as the “boiling crisis”. Since beyond the CHF point the heat transfer coefficient decreases, the transition to film boiling is usually inevitable.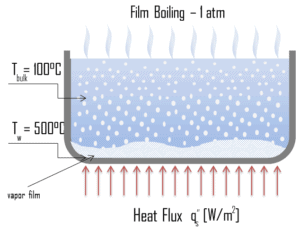 Film Boiling. A further increase in the heat flux causes a film of vapour to cover the surface. This significantly reduces the convection coefficient, since the vapor layer has significantly lower heat transfer ability. As a result the excess temperature shoots up to a very high value. Beyond the Leidenfrost point, a continuous vapor film blankets the surface and there is no contact between the liquid phase and the surface. In this situation the heat transfer is both by radiation and by conduction to the vapour. If the material is not strong enough for withstanding this temperature, the equipment will fail by damage to the material. This phenomenon is also known as burn out. In pressurized water reactors, one of key safety requirements (maybe the most important) is that a departure from nucleate boiling (DNB) will not occur during steady state operation, normal operational transients, and anticipated operational occurrences (AOOs). Fuel cladding integrity will be maintained if the minimum DNBR remains above the 95/95 DNBR limit for PWRs ( a 95% probability at a 95% confidence level). Since this phenomenon deteriorates the heat transfer coefficient and the heat flux remains, heat then accumulates in the fuel rod causing dramatic rise of cladding and fuel temperature. Simply, a very high temperature difference is required to transfer the critical heat flux being produced from the surface of the fuel rod to the reactor coolant (through vapor layer).
Film Boiling. A further increase in the heat flux causes a film of vapour to cover the surface. This significantly reduces the convection coefficient, since the vapor layer has significantly lower heat transfer ability. As a result the excess temperature shoots up to a very high value. Beyond the Leidenfrost point, a continuous vapor film blankets the surface and there is no contact between the liquid phase and the surface. In this situation the heat transfer is both by radiation and by conduction to the vapour. If the material is not strong enough for withstanding this temperature, the equipment will fail by damage to the material. This phenomenon is also known as burn out. In pressurized water reactors, one of key safety requirements (maybe the most important) is that a departure from nucleate boiling (DNB) will not occur during steady state operation, normal operational transients, and anticipated operational occurrences (AOOs). Fuel cladding integrity will be maintained if the minimum DNBR remains above the 95/95 DNBR limit for PWRs ( a 95% probability at a 95% confidence level). Since this phenomenon deteriorates the heat transfer coefficient and the heat flux remains, heat then accumulates in the fuel rod causing dramatic rise of cladding and fuel temperature. Simply, a very high temperature difference is required to transfer the critical heat flux being produced from the surface of the fuel rod to the reactor coolant (through vapor layer).
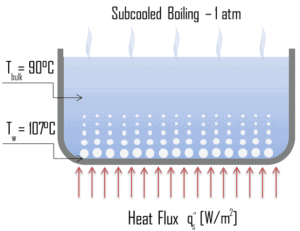 Categorization by the subcooling temperature, ΔTsub.
Categorization by the subcooling temperature, ΔTsub.
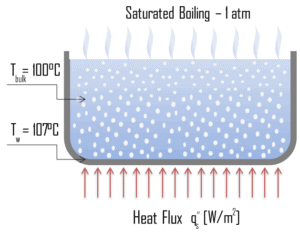
Boiling may also be classified according to whether it is subcooled or saturated:
- Subcooled boiling. In subcooled boiling, the temperature of most of the liquid is below the saturation temperature and bubbles formed at the surface may condense in the liquid. This condensation (collapsing) produces a sound of frequency ~ 100Hz – 1 KHz. This is why an electric kettle makes the most noise before the water comes to saturated boiling. The term subcooling refers to a liquid existing at a temperature below its normal boiling point.
- Saturated Boiling. In saturated boiling (known also as bulk boiling), the temperature of the liquid slightly exceeds the saturation temperature. Bulk boiling may occur, when system temperature increases or system pressure drops to boiling point. At this point, the bubbles entering the coolant channel will not collapse. The bubbles will tend to join together and form bigger steam bubbles. Steam bubbles are then propelled through the liquid by buoyancy forces, eventually escaping from a free surface.
We hope, this article, Boiling water – How to boil water, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.