Triple Point of Water
Source: wikipedia.org CC BY-SA
In thermodynamics, the triple point of a substance is the unique combination of temperature and pressure at which solid phase, liquid phase, and gaseous phase can all coexist in thermodynamic equilibrium.
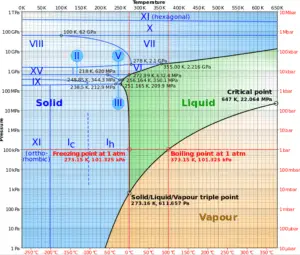
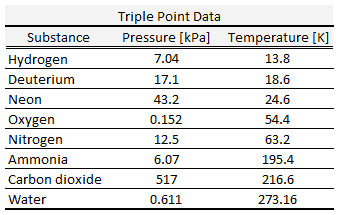
By international agreement, the triple point of water has been assigned a value of 273.16 K (0.01 °C; 32.02 °F) and a partial vapor pressure of 611.66 pascals (6.1166 mbar; 0.0060366 atm). At that point, it is possible to change all of the substance to vapor, water, or ice by making arbitrarily small changes in pressure and temperature. Even if the total pressure is well above the triple point of water, provided that the partial pressure of the water vapor is 611.657 pascals, then the system can still be brought to the triple point of water.
The triple point of water, T3 = 273.16 K, is the standard fixed-point temperature for the calibration of thermometers. This agreement also sets the size of the kelvin as 1/273.16 of the difference between the triple-point temperature of water and absolute zero.
The phase diagram of water is a pressure-temperature diagram for water that shows how all three phases (solid, liquid, and vapor) may coexist together in thermal equilibrium. Along the vaporization line, the liquid and vapor phases are in equilibrium, along the fusion line, the solid and liquid phases are in equilibrium and along the sublimation line, the solid and vapor phases are in equilibrium. The only point at which all three phases may exist in equilibrium is the triple point.
The triple point of water corresponds to the minimum pressure at which water in the liquid state can exist. At pressures below the triple point (as in outer space), solid ice when heated at constant pressure is converted directly into water vapor in a process known as sublimation. In general, sublimation is a phase change of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase. Above the triple point, solid ice when heated at constant pressure first melts to form liquid water, and then evaporates to form water vapor.
We hope, this article, Triple Point of Water, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.