Direction of Thermodynamic Processes
 Many thermodynamic processes proceed naturally in one direction but not the opposite. For example, when a temperature difference does exist heat flows spontaneously from the warmer system to the colder system, never the reverse. In fact, such heat flow (from a colder body to a warmer system) would not violate the first law of thermodynamics, i.e. energy would be conserved. But it doesn’t happen in nature.
Many thermodynamic processes proceed naturally in one direction but not the opposite. For example, when a temperature difference does exist heat flows spontaneously from the warmer system to the colder system, never the reverse. In fact, such heat flow (from a colder body to a warmer system) would not violate the first law of thermodynamics, i.e. energy would be conserved. But it doesn’t happen in nature.
For example, burning gasoline to power cars is an energy conversion process we rely on. The chemical energy in gasoline is converted to thermal energy, which is then converted to mechanical energy that makes the car move. The mechanical energy has been converted to kinetic energy. When we use the brakes to stop a car, that kinetic energy is converted by friction back to heat, or thermal energy. In this reverse direction, there are plenty of devices that convert heat partially into mechanical energy. But you cannot build a machine that converts heat completely into mechanical energy. There will always be significant energy losses.
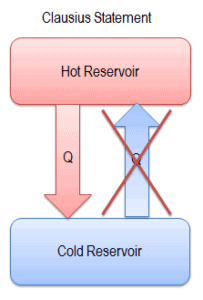
Directions of thermodynamic processes are subject of the second law of thermodynamics, especially of the Clausius Statement of the Second Law.
“It is impossible to construct a device which operates on a cycle and whose sole effect is the transfer of heat from a cooler body to a hotter body”.
Heat cannot spontaneously flow from cold system to hot system without external work being performed on the system. This is exactly what refrigerators and heat pumps accomplish. In a refrigerator, heat flows from cold to hot, but only when forced by an external work, refrigerators are driven by electric motors requiring work from their surroundings to operate.
The Clausius and the Kelvin-Planck statements have been shown to be equivalent.
We hope, this article, Direction of Thermodynamic Processes, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.