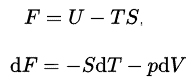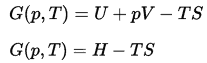Thermodynamic Potentials
Thermodynamic potentials are scalar quantities used to represent state functions. Together with the corresponding equations of state, thermodynamic potentials describe the equilibrium behavior of a system as a function of so-called ”natural variables”.
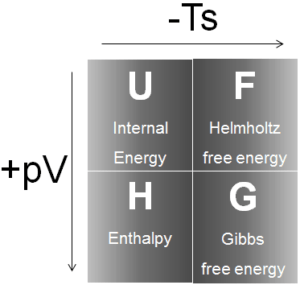
Four common thermodynamic potentials are:
Internal Energy
In thermodynamics, internal energy (also called the thermal energy) is defined as the energy associated with microscopic forms of energy. It is an extensive quantity, it depends on the size of the system, or on the amount of substance it contains. The SI unit of internal energy is the joule (J). It is the energy contained within the system, excluding the kinetic energy of motion of the system as a whole and the potential energy of the system. Microscopic forms of energy include those due to the rotation, vibration, translation, and interactions among the molecules of a substance. None of these forms of energy can be measured or evaluated directly, but techniques have been developed to evaluate the change in the total sum of all these microscopic forms of energy.
In addition, energy is can be stored in the chemical bonds between the atoms that make up the molecules. This energy storage on the atomic level includes energy associated with electron orbital states, nuclear spin, and binding forces in the nucleus.
Enthalpy
In thermodynamics, the enthalpy is a measurement of energy in a thermodynamic system. It is the thermodynamic quantity equivalent to the total heat content of a system. The enthalpy is defined to be the sum of the internal energy E plus the product of the pressure p and volume V. In many thermodynamic analyses the sum of the internal energy U and the product of pressure p and volume V appears, therefore it is convenient to give the combination a name, enthalpy, and a distinct symbol, H.
H = U + pV
The enthalpy is the preferred expression of system energy changes in many chemical, biological, and physical measurements at constant pressure. It is so useful that it is tabulated in the steam tables along with specific volume and specific internal energy. It is due to the fact, it simplifies the description of energy transfer. At constant pressure, the enthalpy change equals the energy transferred from the environment through heating (Q = H2 – H1) or work other than expansion work. For a variable-pressure process, the difference in enthalpy is not quite as obvious.
Helmholtz Free Energy
In thermodynamics, the Helmholtz free energy is a thermodynamic potential that is defined as the internal energy of the system minus the product of the temperature times the entropy of the system. it measures the “useful” work obtainable from a closed thermodynamic system at a constant volume and pressure.The Helmhotz free energy is defined as:
The internal energy, U, has an exact physical meaning, it is the sum of all the kinetic and potential energies of all the particles in the system. The second term is the amount of spontaneous energy transfer, TS, where S is the final entropy of the system. For a constant temperature process the Helmholtz free energy gives all the reversible work. When a physicists say “free energy” without indicating Helmholtz or Gibbs, they usually means Helmholtz free energy, on the other hand, when a chemists say “free energy” they almost always means Gibbs free energy.
Gibbs Free Energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system. Since the enthalpy is defined to be the sum of the internal energy E plus the product of the pressure p and volume V, the Gibbs free energy is defined as:
The change in Gibbs free energy, ΔG, in chemistry, is a very useful parameter. It can be thought of as the maximum amount of work obtainable from a reaction.
We hope, this article, Thermodynamic Potential, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.