Extensive Properties – Intesive Properties
Thermodynamic properties can be divided into two general classes:
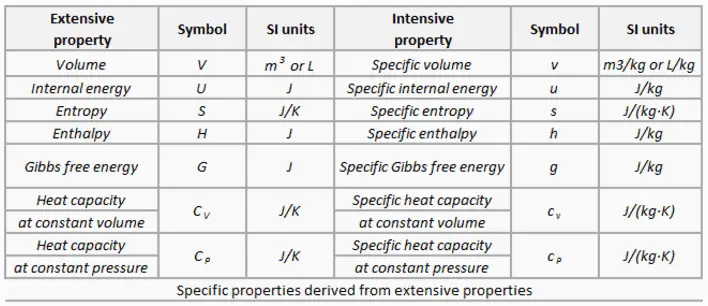
- Extensive properties: An extensive property is dependent upon the amount of mass present or upon the size or extent of a system. Mass, total volume and energy are examples of extensive properties. The value of an extensive property varies directly with the mass. Thus, if a quantity of matter in a given state is divided into two equal parts, each part will have the same value of intensive property as the original and half the value of the extensive property. Extensive properties are additive for subsystems. The system could be divided into any number of subsystems and the value of the property for the system would be the sum of the property for each subsystem.
- Intensive property: An intensive property is independent of the amount of mass and may vary from place to place within the system at any moment. For example, the temperature of a system in thermal equilibrium is the same as the temperature of any part of it. If the system is divided the temperature of each subsystem is identical. Temperature, pressure, specific volume, and density are examples of intensive properties. Specific quantities are also referred to as intensive variables, though there are some intensive variables that have no extensive counterpart, such as pressure or temperature. Intensive properties may be functions of both position and time, whereas extensive properties vary at most with time.
Specific Properties
Specific properties of material are derived from other intensive and extensive properties of that material. For example, the density of water is an intensive property and can be derived from measurements of the mass of a water volume (an extensive property) divided by the volume (another extensive property). Also heat capacity, which is an extensive property of a system can be derived from heat capacity, Cp, and the mass of the system. Dividing these extensive properties gives the specific heat capacity, cp, which is an intensive property.
Specific properties are often used in reference tables as a means of recording material data in a manner that is independent of size or mass. They are very useful for making comparisons about one attribute while cancelling out the effect of variations in another attribute.
We hope, this article, Extensive and Intensive Property, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.