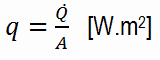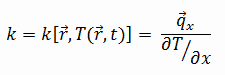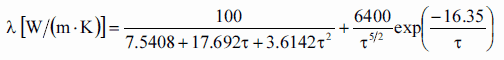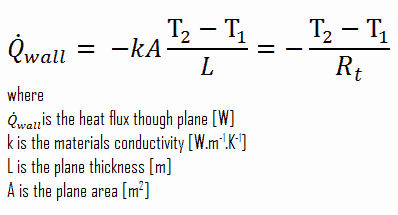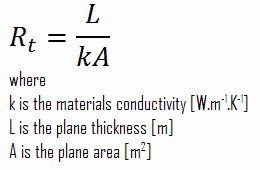Fourier’s law of Thermal Conduction
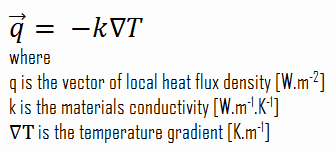
Heat transfer processes can be quantified in terms of appropriate rate equations. The rate equation in this heat transfer mode is based on Fourier’s law of thermal conduction. This law states that the time rate of heat transfer through a material is proportional to the negative gradient in the temperature and to the area, at right angles to that gradient, through which the heat flows. Its differential form is:
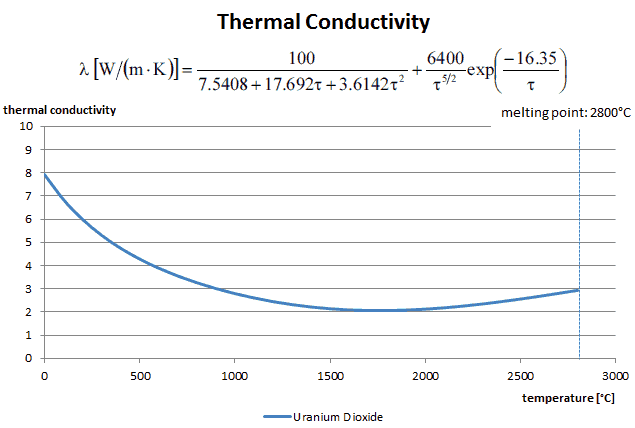
As can be seen, to solve the Fourier’s law we have to involve the temperature difference, the geometry, and the thermal conductivity of the object. This law was first formulated by Joseph Fourier in 1822 who concluded that “the heat flux resulting from thermal conduction is proportional to the magnitude of the temperature gradient and opposite to it in sign”.
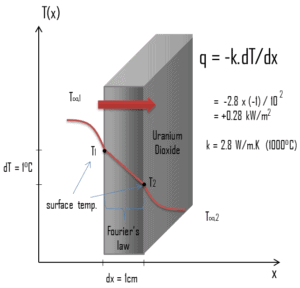
Similarly as the Fourier’s law determines the heat flux through a slab, it can also be used to determine the temperature difference, when q is known. This can be used for calculation of the temperature in the centre of fuel pellet as will be shown in following sections.
Example – Heat flux through a window
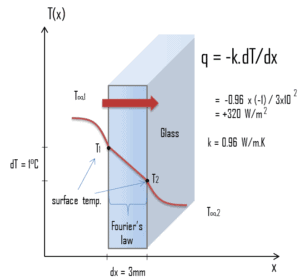
A major source of heat loss from a house is through the windows. Calculate the rate of heat flux through a glass window 1.5 m x 1.0 m in area and 3.0 mm thick, if the temperatures at the inner and outer surfaces are 14.0°C and 13.0°C, respectively. Calculate the heat flux through this window.
Solution:
At this point, we know the temperatures at the surfaces of material. These temperatures are given also by conditions inside the house and outside the house. In this case, heat flows by conduction through the glass from the higher inside temperature to the lower outside temperature. We use the Fourier’s law of thermal conduction equation:
We assume that the thermal conductivity of a common glass is k = 0.96 W/m.K.
The heat flux will then be:
q = 0.96 [W/m.K] x 1 [K] / 3.0 x 10-3 [m] = 320 W/m2
The total heat loss through this window will be:
qloss = q . A = 320 x 1.5 x 1.0 = 480W
Fourier’s Law and Thermal Resistance
 Thermal resistance is the reciprocal of thermal conductance. Just as an electrical resistance is associated with the conduction of electricity, a thermal resistance may be associated with the conduction of heat.
Thermal resistance is the reciprocal of thermal conductance. Just as an electrical resistance is associated with the conduction of electricity, a thermal resistance may be associated with the conduction of heat.
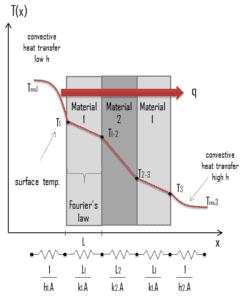
Consider a plane wall of thickness L and average thermal conductivity k. The two surfaces of the wall are maintained at constant temperatures of T1 and T2. For one-dimensional steady heat conduction through the wall, we have T(x). Then Fourier’s law of heat conduction for the wall can be expressed as:
Thermal resistance is a heat property and a measurement of a temperature difference by which an object or material resists a heat flow. The thermal resistance for conduction in a plane wall is defined as:
We hope, this article, Fourier’s Law of Thermal Conduction, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.