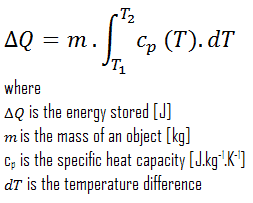Heat Absorption
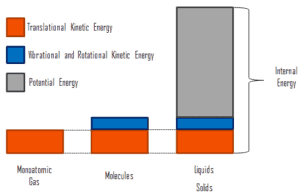 In thermodynamics, internal energy (also called the thermal energy) is defined as the energy associated with microscopic forms of energy. It is an extensive quantity, it depends on the size of the system, or on the amount of substance it contains. The SI unit of internal energy is the joule (J). It is the energy contained within the system, excluding the kinetic energy of motion of the system as a whole and the potential energy of the system. Microscopic forms of energy include those due to the rotation, vibration, translation, and interactions among the molecules of a substance. None of these forms of energy can be measured or evaluated directly, but techniques have been developed to evaluate the change in the total sum of all these microscopic forms of energy.
In thermodynamics, internal energy (also called the thermal energy) is defined as the energy associated with microscopic forms of energy. It is an extensive quantity, it depends on the size of the system, or on the amount of substance it contains. The SI unit of internal energy is the joule (J). It is the energy contained within the system, excluding the kinetic energy of motion of the system as a whole and the potential energy of the system. Microscopic forms of energy include those due to the rotation, vibration, translation, and interactions among the molecules of a substance. None of these forms of energy can be measured or evaluated directly, but techniques have been developed to evaluate the change in the total sum of all these microscopic forms of energy.
Heat Absorption
In addition, energy is can be stored in the chemical bonds between the atoms that make up the molecules. This energy storage on the atomic level includes energy associated with electron orbital states, nuclear spin, and binding forces in the nucleus.

Thermal energy can be also very effectively absorbed and stored. Nowadays, situation on energy markets is different. The increasing on the prices of the conventional energy sources and the environmental awareness have leaded to increase the use of renewable energies and the energy efficiency. Thermal energy storage forms a key component of a power plant for improvement of its dispatchability, especially for concentrating solar power plants (CSP). Thermal energy storage (TES) is achieved with widely differing technologies. There are three methods used and still being investigated in order to store thermal energy.
- Sensible Heat Storage (SHS)
- Latent Heat Storage (LHS)
- Thermo-chemical Storage
Absorption in Sensible Heat
The most direct way is the storage of sensible heat. Sensible heat storage is based on raising the temperature of a liquid or solid to store heat and releasing it with the decrease of temperature when it is required. The volumes needed to store energy in the scale that world needs are extremely large. Materials used in sensible heat storage must have high heat capacity and also high boiling or melting point. Although this method of heat storage is currently less efficient for heat storage, it is least complicated compared with latent or chemical heat and it is inexpensive.
From thermodynamics point of view, the storage of sensible heat is based on the increase of enthalpy of the material in the store, either a liquid or a solid in most cases. The sensible effect is a change in temperature. Heat stored can be obtained by the equation:
Absorption in Latent Heat
A common approach to thermal energy storage is to use materials known as phase change materials (PCMs). These materials store heat when they undergo a phase change, for example, from solid to liquid, from liquid to gas or from solid to solid (change of one crystalline form into another without a physical phase change).
The phase change “solid-to-liquid” is the most used, but also solid-to-solid change is of interest. These materials can be used as an effective way of storing thermal energy (solar energy, off-peak electricity, industrial waste heat). In comparison to sensible heat storage systems, the latent heat storage has the advantages of high storage density (due to high latent heat of fusion) and the isothermal nature of the storage process. The heat of fusion or the heat of evaporation is much greater than the specific heat capacity. The comparison between latent heat storage and sensible heat storage shows that in latent heat storage storage densities are typically 5 to 10 times higher.
In general, latent heat effects associated with the phase change are significant. Latent heat, known also as the enthalpy of vaporization (liquid-to-vapor phase change) or enthalpy of fusion (solid-to-liquid phase change), is the amount of heat added to or removed from a substance to produce a change in phase. This energy breaks down the intermolecular attractive forces, and also must provide the energy necessary to expand the substance (the pΔV work). When latent heat is added, no temperature change occurs.
Absorption in Chemical Energy
One of three possible approaches to thermal energy storage is to use reversible thermo-chemical reactions. The most important advantage of the thermo-chemical storage method is that the enthalpy of reaction is considerably larger than the specific heat or the heat of fusion. Therefore the storage density is much better. In chemical reactions, energy is stored in the chemical bonds between the atoms that make up the molecules. Energy storage on the atomic level includes energy associated with electron orbital states. Whether a chemical reaction absorbs or releases energy, there is no overall change in the amount of energy during the reaction. That’s because of the law of conservation of energy, which states that:
Energy cannot be created or destroyed. Energy may change form during a chemical reaction.
One example of an experimental storage system based on chemical reaction energy is the salt hydrate technology. The system is especially advantageous for seasonal thermal energy storage. The system uses the reaction energy created when salts are hydrated or dehydrated. It works by storing heat in a container containing 50% sodium hydroxide (NaOH) solution. Heat (e.g. from using a solar collector) is stored by evaporating the water in an endothermic reaction. When water is added again, heat is released in an exothermic reaction at 50 °C. Current systems operate at 60% efficiency.
Radiant Heat Absorption
 In preceding chapters, we have discussed convection and conduction, which require the presence of matter as a medium to carry the heat from the hotter to the colder region. But a third type of heat transfer, radiation heat transfer, occurs without any medium at all. In general, the radiation heat transferfrom one surface to another is the radiation leaving the first surface for the other minus that arriving from the second surface. Radiation heat transfer is mediated by electromagnetic radiation, known as thermal radiation, that arises due to the temperature of a body.
In preceding chapters, we have discussed convection and conduction, which require the presence of matter as a medium to carry the heat from the hotter to the colder region. But a third type of heat transfer, radiation heat transfer, occurs without any medium at all. In general, the radiation heat transferfrom one surface to another is the radiation leaving the first surface for the other minus that arriving from the second surface. Radiation heat transfer is mediated by electromagnetic radiation, known as thermal radiation, that arises due to the temperature of a body.
From its definition, a blackbody, which is an idealized physical body, absorbs all incident electromagnetic radiation, regardless of frequency or angle of incidence. That is, a blackbody is a perfect absorber. Since for real objects the absorptivity is less than unity, a real object can not absorb all incident light. The incomplete absorption can be due to some of the incident light being transmitted through the body or to some of it being reflected at the surface of the body.
In general, the absorptivity and the emissivity are interconnected by the Kirchhoff’s Law of thermal radiation, which states:
For an arbitrary body emitting and absorbing thermal radiation in thermodynamic equilibrium, the emissivity is equal to the absorptivity.
emissivity ε = absorptivity α
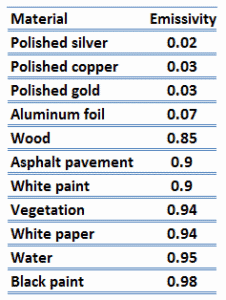
Note that visible radiation occupies a very narrow band of the spectrum from 400 to 760 nm, we cannot make any judgments about the blackness of a surface on the basis of visual observations. For example, consider white paper that reflects visible light and thus appear white. On the other hand it is essentially black for infrared radiation (absorptivity α = 0.94) since they strongly absorb long-wavelength radiation.
We hope, this article, Heat Absorption, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.