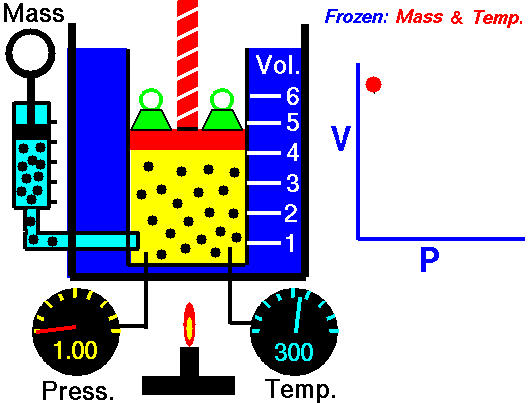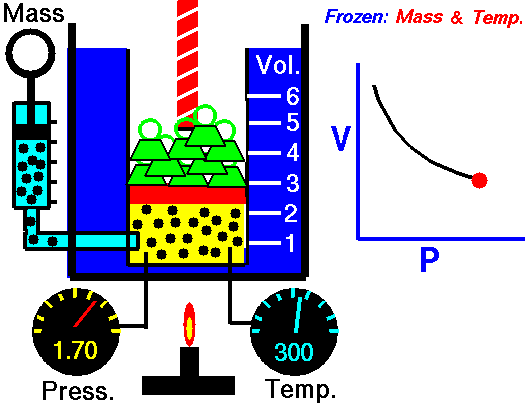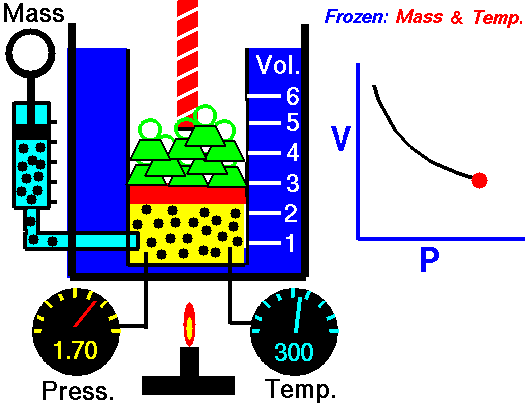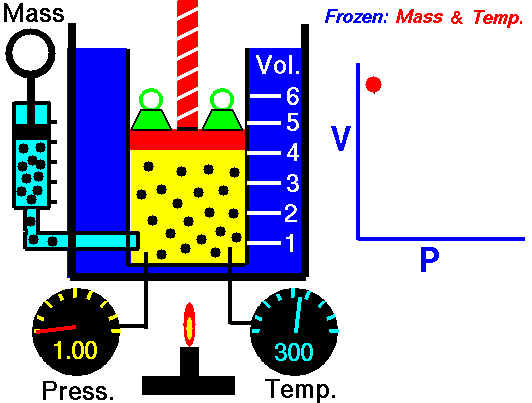
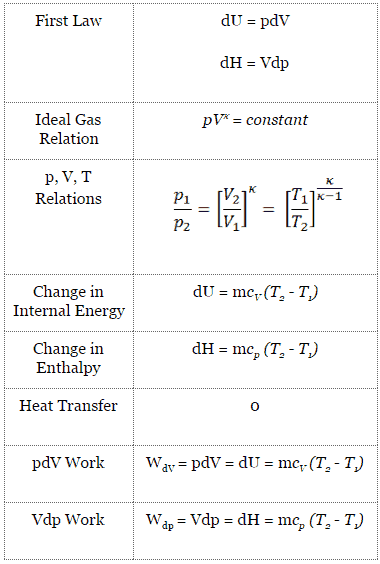Isothermal Process
[/su_accordion]An isothermal process is a thermodynamic process, in which the temperature of the system remains constant (T = const). The heat transfer into or out of the system typically must happen at such a slow rate in order to continually adjust to the temperature of the reservoir through heat exchange. In each of these states the thermal equilibrium is maintained.For an ideal gas and a polytropic process, the case n = 1 corresponds to an isothermal (constant-temperature) process. In contrast to adiabatic process , in which n = κ and a system exchanges no heat with its surroundings (Q = 0; ∆T≠0), in an isothermal process there is no change in the internal energy (due to ∆T=0) and therefore ΔU = 0 (for ideal gases) and Q ≠ 0. An adiabatic process is not necessarily an isothermal process, nor is an isothermal process necessarily adiabatic.
In engineering, phase changes, such as evaporation or melting, are isothermal processes when, as is usually the case, they occur at constant pressure and temperature.
Isothermal Process and the First Law
The classical form of the first law of thermodynamics is the following equation:
dU = dQ – dW
In this equation dW is equal to dW = pdV and is known as the boundary work.
In isothermal process and the ideal gas, all heat added to the system will be used to do work:
Isothermal process (dU = 0):
dU = 0 = Q – W → W = Q (for ideal gas)
The isothermal process can be expressed with the ideal gas law as:
pV = constant
or
p1V1 = p2V2
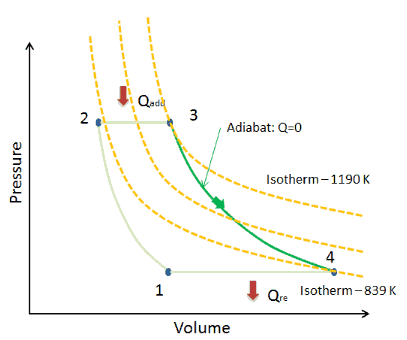
On a p-V diagram, the process occurs along a line (called an isotherm) that has the equation p = constant / V.
Adiabatic Process
[/su_accordion]An adiabatic process is a thermodynamic process, in which there is no heat transfer into or out of the system (Q = 0). The system can be considered to be perfectly insulated. In an adiabatic process, energy is transferred only as work. The assumption of no heat transfer is very important, since we can use the adiabatic approximation only in very rapid processes. In these rapid processes, there is not enough time for the transfer of energy as heat to take place to or from the system.In real devices (such as turbines, pumps, and compressors) heat losses and losses in the combustion process occur, but these losses are usually low in comparison to overall energy flow and we can approximate some thermodynamic processes by the adiabatic process.
Adiabatic Process and the First Law
For a closed system, we can write the first law of thermodynamics in terms of enthalpy:
dH = dQ + Vdp
In this equation the term Vdp is a flow process work. This work, Vdp, is used for open flow systems like a turbine or a pump in which there is a “dp”, i.e. change in pressure. As can be seen, this form of the law simplifies the description of energy transfer. In adiabatic process, the enthalpy change equals the flow process work done on or by the system:
Adiabatic process (dQ = 0):
dH = Vdp → W = H2 – H1 → H2 – H1 = Cp (T2 – T1) (for an ideal gas)
Free Expansion – Joule Expansion
These are adiabatic processes in which no transfer of heat occurs between the system and its environment and no work is done on or by the system. These types of adiabatic processes are called free expansion. It is an irreversible process in which a gas expands into an insulated evacuated chamber. It is also called Joule expansion. For an ideal gas, the temperature doesn’t change (this means that the process is also isothermal), however, real gases experience a temperature change during free expansion. In free expansion Q = W = 0, and the first law requires that:
dEint = 0
A free expansion can not be plotted on a P-V diagram, because the process is rapid, not quasistatic. The intermediate states are not equilibrium states, and hence the pressure is not clearly defined.
We hope, this article, Isothermal Process and Adiabatic Process, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.