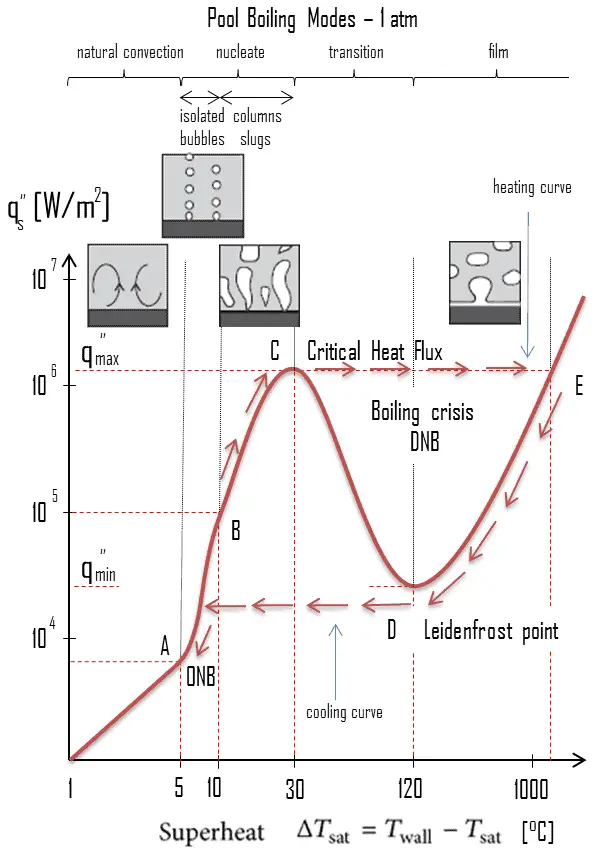
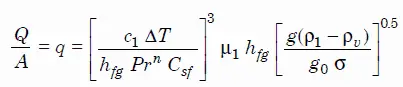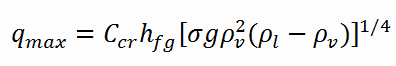Pool Boiling – Boiling Curve
 As was written, the most common configuration, known as pool boiling is when a pool of liquid is heated from below through a horizontal surface. In pool boiling the liquid is quiescent and its motion near the surface is primarily due to natural convection and to mixing induced by bubble growth and detachment.
As was written, the most common configuration, known as pool boiling is when a pool of liquid is heated from below through a horizontal surface. In pool boiling the liquid is quiescent and its motion near the surface is primarily due to natural convection and to mixing induced by bubble growth and detachment.
The pioneering work on boiling was done in 1934 by S. Nukiyama, who used electrically heated nichrome and platinum wires immersed in liquids in his experiments. Nukiyama was the first to identify different regimes of pool boiling using his apparatus. He noticed that boiling takes different forms, depending on the value of the wall superheat temperature ΔTsat (known also as the excess temperature), which is defined as the difference between the wall temperature, Twall and the saturation temperature, Tsat.
Four different boiling regimes of pool boiling (based on the excess temperature) are observed:
- Natural Convection Boiling ΔTsat < 5°C
- Nucleate Boiling 5°C < ΔTsat < 30°C
- Transition Boiling 30°C < ΔTsat < 200°C
- Film Boiling 200°C < ΔTsat
These regimes are illustrated on the boiling curve in the figure, which is a plot of heat flux versus the excess temperature. Although the boiling curve given in this figure is for water, the general shape of the boiling curve remains the same for different coolants. Note that, the specific shape of the curve depends also on the system parameters such as the pressure and coolant flow rate, but it is practically independent of the geometry of the heating surface.
Nucleate Boiling
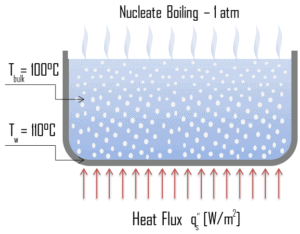 The most common type of local boiling encountered in nuclear facilities is nucleate boiling. But in case of nuclear reactors, nucleate boiling occurs at significant flow rates through the reactor. In nucleate boiling, steam bubbles form at the heat transfer surface and then break away and are carried into the main stream of the fluid. Such movement enhances heat transfer because the heat generated at the surface is carried directly into the fluid stream. Once in the main fluid stream, the bubbles collapse because the bulk temperature of the fluid is not as high as the heat transfer surface temperature where the bubbles were created. As was written, nucleate boiling at the surface effectively disrupts this stagnant layer and therefore nucleate boiling significantly improves the ability of a surface to transfer thermal energy to bulk fluid. This heat transfer process is sometimes desirable because the energy created at the heat transfer surface is quickly and efficiently “carried” away.
The most common type of local boiling encountered in nuclear facilities is nucleate boiling. But in case of nuclear reactors, nucleate boiling occurs at significant flow rates through the reactor. In nucleate boiling, steam bubbles form at the heat transfer surface and then break away and are carried into the main stream of the fluid. Such movement enhances heat transfer because the heat generated at the surface is carried directly into the fluid stream. Once in the main fluid stream, the bubbles collapse because the bulk temperature of the fluid is not as high as the heat transfer surface temperature where the bubbles were created. As was written, nucleate boiling at the surface effectively disrupts this stagnant layer and therefore nucleate boiling significantly improves the ability of a surface to transfer thermal energy to bulk fluid. This heat transfer process is sometimes desirable because the energy created at the heat transfer surface is quickly and efficiently “carried” away.
Close to the wall the situation is complex for several mechanisms increase the heat flux above that for pure conduction through the liquid.
- Note that, even in turbulent flow, there is a stagnant fluid film layer (laminar sublayer), that isolates the surface of the heat exchanger. The upward flux (due to buoyant forces) of vapor away from the wall must be balanced by an equal mass flux of liquid and this brings cooler liquid into closer proximity to the wall.
- The formation and movement of the bubbles turbulises the liquid near the wall and thus increases heat transfer from the wall to the liquid.
- Boiling differ from other forms of convection in that it depends on the latent heat of vaporization, which is very high for common pressures, therefore large amounts of heat can be transferred during boiling essentially at constant temperature.
The nucleate boiling heat flux cannot be increased indefinitely. At some value, we call it the “critical heat flux” (CHF), the steam produced can form an insulating layer over the surface, which in turn deteriorates the heat transfer coefficient. This is because a large fraction of the surface is covered by a vapor film, which acts as an thermal insulation due to the low thermal conductivity of the vapor relative to that of the liquid. Immediately after the critical heat flux has been reached, boiling become unstable and transition boiling occurs. The transition from nucleate boiling to film boiling is known as the “boiling crisis”. Since beyond the CHF point the heat transfer coefficient decreases, the transition to film boiling is usually inevitable.
Boiling Crisis – Critical Heat Flux
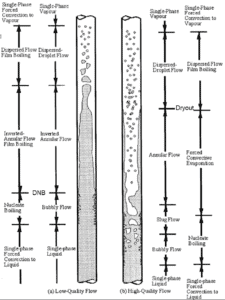 As was written, in nuclear reactors, limitations of the local heat flux is of the highest importance for reactor safety. For pressurized water reactors and also for boiling water reactors, there are thermal-hydraulic phenomena, which cause a sudden decrease in the efficiency of heat transfer (more precisely in the heat transfer coefficient). These phenomena occur at certain value of heat flux, known as the “critical heat flux”. The phenomena, that cause the deterioration of heat transfer are different for PWRs and for BWRs.
As was written, in nuclear reactors, limitations of the local heat flux is of the highest importance for reactor safety. For pressurized water reactors and also for boiling water reactors, there are thermal-hydraulic phenomena, which cause a sudden decrease in the efficiency of heat transfer (more precisely in the heat transfer coefficient). These phenomena occur at certain value of heat flux, known as the “critical heat flux”. The phenomena, that cause the deterioration of heat transfer are different for PWRs and for BWRs.
In both types of reactors, the problem is more or less associated with departure from nucleate boiling. The nucleate boiling heat flux cannot be increased indefinitely. At some value, we call it the “critical heat flux” (CHF), the steam produced can form an insulating layer over the surface, which in turn deteriorates the heat transfer coefficient. Immediately after the critical heat flux has been reached, boiling become unstable and film boiling occurs. The transition from nucleate boiling to film boiling is known as the “boiling crisis”. As was written, the phenomena, that cause the deterioration of heat transfer are different for PWRs and for BWRs.
We hope, this article, Pool Boiling – Boiling Curve, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.
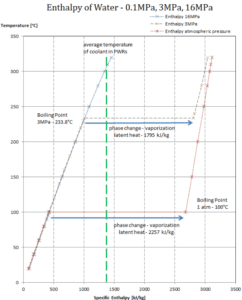
 The change from the liquid to the vapor state due to boiling is sustained by heat transfer from the solid surface; conversely, condensation of a vapor to the liquid state results in heat transfer to the solid surface. Boiling and condensation differ from other forms of convection in that they depend on the latent heat of vaporization, which is very high for common
The change from the liquid to the vapor state due to boiling is sustained by heat transfer from the solid surface; conversely, condensation of a vapor to the liquid state results in heat transfer to the solid surface. Boiling and condensation differ from other forms of convection in that they depend on the latent heat of vaporization, which is very high for common