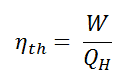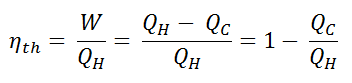Thermal Efficiency for Atkinson Cycle
In general the thermal efficiency, ηth, of any heat engine is defined as the ratio of the work it does, W, to the heat input at the high temperature, QH.
The thermal efficiency, ηth, represents the fraction of heat, QH, that is converted to work. Since energy is conserved according to the first law of thermodynamics and energy cannot be be converted to work completely, the heat input, QH, must equal the work done, W, plus the heat that must be dissipated as waste heat QC into the environment. Therefore we can rewrite the formula for thermal efficiency as:
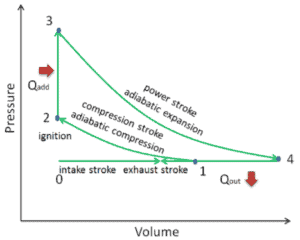
The heat absorbed occurs during combustion of fuel-air mixture, when the spark occurs, roughly at constant volume. Since during an isochoric process there is no work done by or on the system, the first law of thermodynamics dictates ∆U = ∆Q.
Therefore the heat added and rejected are given by:
Qadd = mcv (T3 – T2)
Qout = mcp (T4 – T1)
Substituting these expressions for the heat added and rejected in the expression for thermal efficiency yields:
Furthermore, it can be derived that in terms of:
- the ratio V1/V2, which is known as the compression ratio – CR
- the ratio V4/V3, which is known as the expansion ratio – ER.
- κ = cp/cv
The expression for thermal efficiency using these characteristics is:
We hope, this article, Thermal Efficiency for Atkinson Cycle, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.