Prandtl Number of Liquid Metals
For liquid metals the Prandtl number is very small, generally in the range from 0.01 to 0.001. This means that the thermal diffusivity, which is related to the rate of heat transfer by conduction, unambiguously dominates. This very high thermal diffusivity results from very high thermal conductivity of metals, which is about 100 times higher than that of water. The Prandtl number for sodium at a typical operating temperature in the Sodium-cooled fast reactors is about 0.004.
The Prandtl number enters many calculations of heat transfer in liquid metal reactors. Two of promising designs of Generation IV reactors use a liquid metal as the reactor coolant. The development of Generation IV reactors represents a challenge from an engineering point of view.
- Sodium-cooled fast reactor
- Lead-cooled fast reactor
One of the main challenges in numerical simulation is the reliable modeling of heat transfer in liquid-metal cooled reactors by Computational Fluid Dynamics (CFD). Heat transfer applications with low-Prandtl number fluids are often in the transition range between conduction and convection dominated regimes.
Prandtl Number
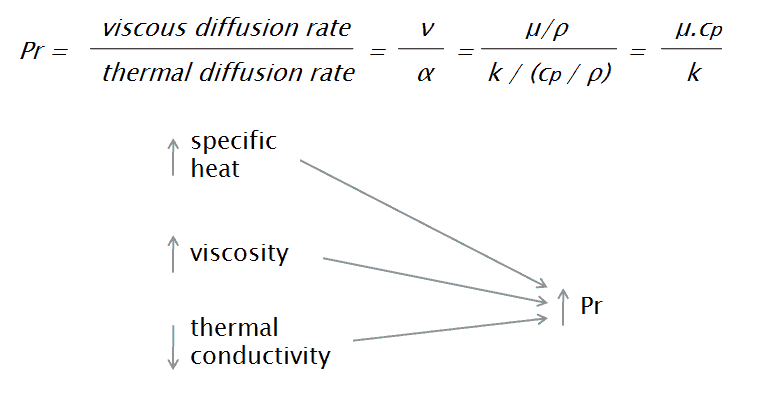
The Prandtl number is a dimensionless number, named after its inventor, a German engineer Ludwig Prandtl, who also identified the boundary layer. The Prandtl number is defined as the ratio of momentum diffusivity to thermal diffusivity. The momentum diffusivity, or as it is normally called, kinematic viscosity, tells us the material’s resistance to shear-flows (different layers of the flow travel with different velocities due to e.g. different speeds of adjacent walls) in relation to density. That is, the Prandtl number is given as:
where:
ν is momentum diffusivity (kinematic viscosity) [m2/s]
α is thermal diffusivity [m2/s]
μ is dynamic viscosity [N.s/m2]
k is thermal conductivity [W/m.K]
cp is specific heat [J/kg.K]
ρ is density [kg/m3]
Small values of the Prandtl number, Pr << 1, means the thermal diffusivity dominates. Whereas with large values, Pr >> 1, the momentum diffusivity dominates the behavior. For example, the typical value for liquid mercury, which is about 0.025, indicates that the heat conduction is more significant compared to convection, so thermal diffusivity is dominant. When Pr is small, it means that the heat diffuses quickly compared to the velocity.
In comparison to Reynolds number, the Prandtl number is not dependent on geometry of an object involved in the problem, but is dependent solely on the fluid and the fluid state. As such, the Prandtl number is often found in property tables alongside other properties such as viscosity and thermal conductivity.
We hope, this article, Prandtl Number of Liquid Metals, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.