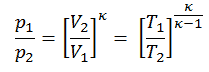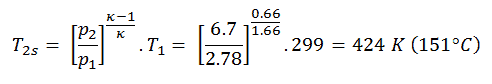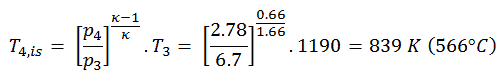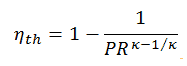Brayton Cycle – Problem with Solution
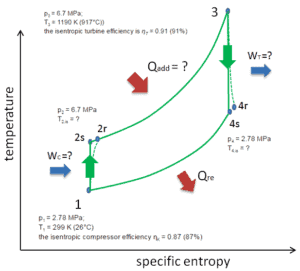
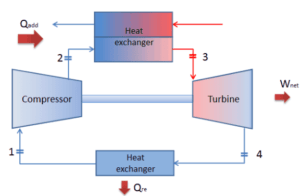 Let assume the closed Brayton cycle, which is the one of most common thermodynamic cycles that can be found in modern gas turbine engines. In this case assume a helium gas turbine with single compressor and single turbine arrangement. One of key parameters of such engines is the maximum turbine inlet temperature and the compressor pressure ratio (PR = p2/p1) which determines the thermal efficiency of such engine.
Let assume the closed Brayton cycle, which is the one of most common thermodynamic cycles that can be found in modern gas turbine engines. In this case assume a helium gas turbine with single compressor and single turbine arrangement. One of key parameters of such engines is the maximum turbine inlet temperature and the compressor pressure ratio (PR = p2/p1) which determines the thermal efficiency of such engine.
In this turbine the high-pressure stage receives gas (point 3 at the figure) from a heat exchanger:
- p3 = 6.7 MPa;
- T3 = 1190 K (917°C))
- the isentropic turbine efficiency is ηT = 0.91 (91%)
and exhaust it to another heat exchanger, where the outlet pressure is (point 4):
- p4 = 2.78 MPa
- T4,is = ?
Thus the compressor pressure ratio is equal to PR = 2.41. Moreover we know, that the compressor receives gas (point 1) at the figure:
- p1 = 2.78 MPa;
- T1 = 299 K (26°C)
- the isentropic compressor efficiency ηK = 0.87 (87%).
The heat capacity ratio, , for helium is equal to =cp/cv=1.66
- the heat added by the heat exchanger (between 2 → 3)
- the compressor outlet temperature of the gas (T2,is)
- the real work done on this compressor, when the isentropic compressor efficiency is ηK = 0.87 (87%)
- the turbine outlet temperature of the gas (T4,is)
- the real work done by this turbine, when the isentropic turbine efficiency is ηT = 0.91 (91%)
- the thermal efficiency of this cycle
Solution:
1) + 2)
From the first law of thermodynamics, the net heat added is given by Qadd,ex = H3 – H2 [kJ] or Qadd = Cp.(T3-T2s), but in this case we do not know the temperature (T2s) at the outlet of the compressor. We will solve this problem in intensive variables. We have to rewrite the previous equation (to include ηK) using the term (+h1 – h1) to:
Qadd = h3 – h2 = h3 – h1 – (h2s – h1)/ηK [kJ/kg]
Qadd = cp(T3-T1) – (cp(T2s-T1)/ηK)
Then we will calculate the temperature, T2s, using p, V, T Relation (from Ideal Gas Law) for adiabatic process between (1 → 2).
In this equation the factor for helium is equal to =cp/cv=1.66. From the previous equation follows that the compressor outlet temperature, T2s, is:
Qadd = cp(T3-T1) – (cp(T2s-T1)/ηK) = 5200.(1190 – 299) – 5200.(424-299)/0.87 = 4.633 MJ/kg – 0.747 MJ/kg = 3.886 MJ/kg
3)
The work done on the gas by the compressor in the isentropic compression process is:
WC,s = cp (T2s – T1) = 5200 x (424 – 299) = 0.650 MJ/kg
The real work done on the gas by the compressor in the adiabatic compression is then:
WC,real = cp (T2s – T1). ηC = 5200 x (424 – 299) / 0.87 = 0.747 MJ/kg
4)
The turbine outlet temperature of the gas, T4,is, can be calculated using the same p, V, T Relation as in 2) but between states 3 and 4:
From the previous equation follows that the outlet temperature of the gas, T4,is, is:
5)
The work done by gas turbine in the isentropic expansion is then:
WT,s = cp (T3 – T4s) = 5200 x (1190 – 839) = 1.825 MJ/kg
The real work done by gas turbine in the adiabatic expansion is then:
WT,real = cp (T3 – T4s) . ηT = 5200 x (1190 – 839) x 0.91 = 1.661 MJ/kg
6)
As was derived in the previous section, the thermal efficiency of an ideal Brayton cycle is a function of pressure ratio and κ:
therefore
ηth = 0.295 = 29.5%
The thermal efficiency can be also calculated using the work and the heat (without ηK):
ηth,s = (WT,s – WC,s) / Qadd,s = (1.825 – 0.650) / 3.983 = 0.295 = 29.5%
Finally, the thermal efficiency including isentropic turbine/compressor efficiency is:
ηth,real = (WT,real – WC,real) / Qadd = (1.661 – 0.747) / 3.886 = 0.235 = 23.5%
We hope, this article, Example of Brayton Cycle – Problem with Solution, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.