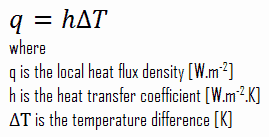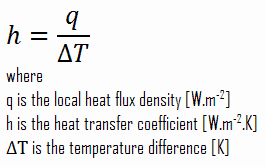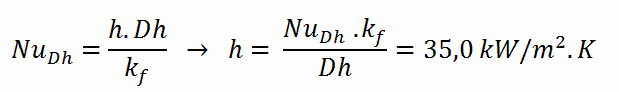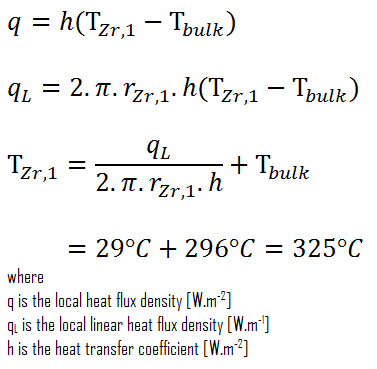Newton’s Law of Cooling
Despite the complexity of convection, the rate of convection heat transfer is observed to be proportional to the temperature difference and is conveniently expressed by Newton’s law of cooling, which states that:
The rate of heat loss of a body is directly proportional to the difference in the temperatures between the body and its surroundings provided the temperature difference is small and the nature of radiating surface remains same.
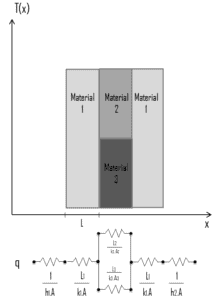
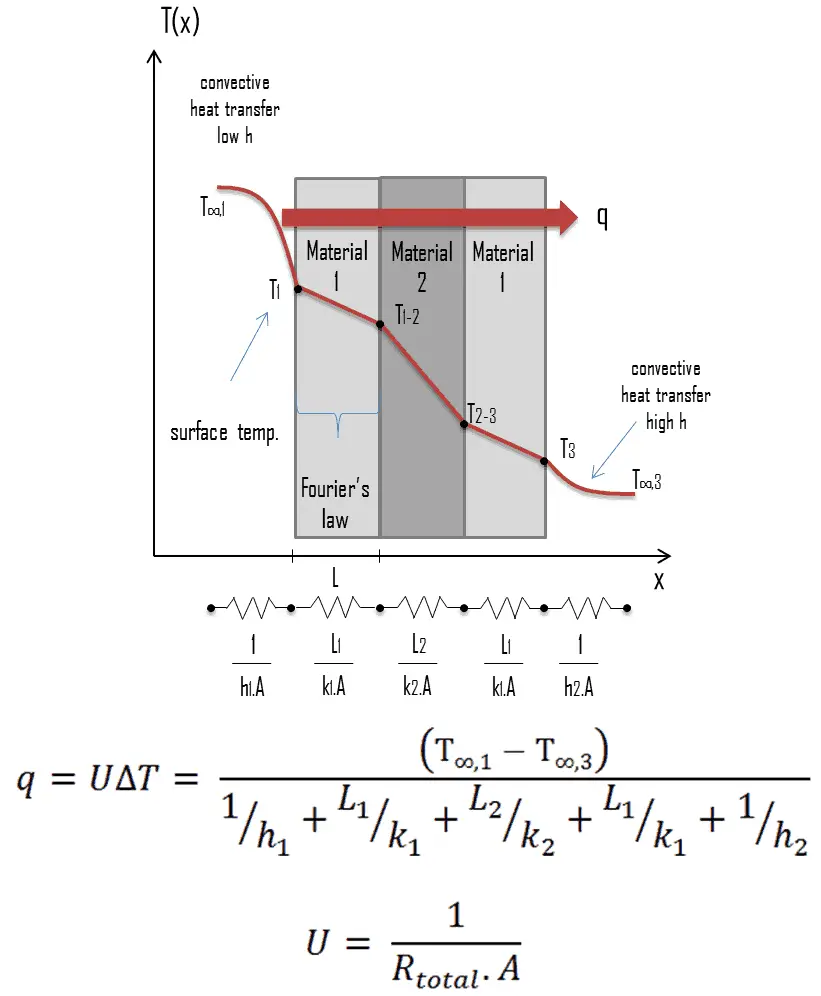
Note that, ΔT is given by the surface or wall temperature, Twall and the bulk temperature, T∞, which is the temperature of the fluid sufficiently far from the surface.
Convective Heat Transfer Coefficient
As can be seen, the constant of proportionality will be crucial in calculations and it is known as the convective heat transfer coefficient, h. The convective heat transfer coefficient, h, can be defined as:
The rate of heat transfer between a solid surface and a fluid per unit surface area per unit temperature difference.
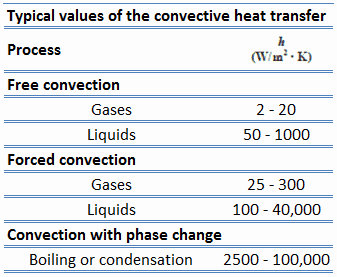
Typically, the convective heat transfer coefficient for laminar flow is relatively low compared to the convective heat transfer coefficient for turbulent flow. This is due to turbulent flow having a thinner stagnant fluid film layer on the heat transfer surface.
It must be noted, this stagnant fluid film layer plays crucial role for the convective heat transfer coefficient. It is observed, that the fluid comes to a complete stop at the surface and assumes a zero velocity relative to the surface. This phenomenon is known as the no-slip condition and therefore, at the surface, energy flow occurs purely by conduction. But in the next layers both conduction and diffusion-mass movement in the molecular level or macroscopic level occurs. Due to the mass movement the rate of energy transfer is higher. As was written, nucleate boiling at the surface effectively disrupts this stagnant layer and therefore nucleate boiling significantly increases the ability of a surface to transfer thermal energy to bulk fluid.
A similar phenomenon occurs for the temperature. It is observed, that the fluid’s temperature at the surface and the surface will have the same temperature at the point of contact. This phenomenon is known as the no-temperature-jump condition and it is very important for theory of nucleate boiling.
Values of the heat transfer coefficient, h, have been measured and tabulated for the commonly encountered fluids and flow situations occurring during heat transfer by convection.
Example: Newton’s Law of Cooling
 From: Example – Convective Heat Transfer
From: Example – Convective Heat Transfer
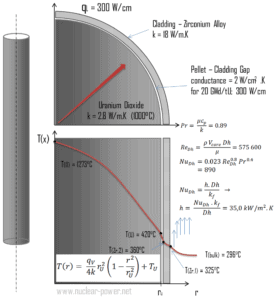
Detailed knowledge of geometry, fluid parameters, outer radius of cladding, linear heat rate, convective heat transfer coefficient allows us to calculate the temperature difference ∆T between the coolant (Tbulk) and the cladding surface (TZr,1).
To calculate the the cladding surface temperature, we have to know:
- the outer diameter of the cladding is: d = 2 x rZr,1 = 9,3 mm
- the Nusselt number, which is NuDh = 890
- the hydraulic diameter of the fuel channel is: Dh = 13,85 mm
- the thermal conductivity of reactor coolant (300°C) is: kH2O = 0.545 W/m.K
- the bulk temperature of reactor coolant at this axial coordinate is: Tbulk = 296°C
- the linear heat rate of the fuel is: qL = 300 W/cm (FQ ≈ 2.0)
The convective heat transfer coefficient, h, is given directly by the definition of Nusselt number:
Finally, we can calculate the cladding surface temperature (TZr,1) simply using the Newton’s Law of Cooling:
For PWRs at normal operation, there is a compressed liquid water inside the reactor core, loops and steam generators. The pressure is maintained at approximately 16MPa. At this pressure water boils at approximately 350°C(662°F). As can be seen, the surface temperature TZr,1 = 325°C ensures, that even subcooled boiling does not occur. Note that, subcooled boiling requires TZr,1 = Tsat. Since the inlet temperatures of the water are usually about 290°C (554°F), it is obvious this example corresponds to the lower part of the core. At higher elevations of the core the bulk temperature may reach up to 330°C. The temperature difference of 29°C causes the subcooled boiling may occur (330°C + 29°C > 350°C). On the other hand, nucleate boiling at the surface effectively disrupts the stagnant layer and therefore nucleate boiling significantly increases the ability of a surface to transfer thermal energy to bulk fluid. As a result, the convective heat transfer coefficient significantly increases and therefore at higher elevations, the temperature difference (TZr,1 – Tbulk) significantly decreases.
We hope, this article, Newton’s Law of Cooling, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about thermal engineering.